TECHNIQUE FOR COLLECTION OF BLOOD SPECIMEN FROM POULTRY BIRD( CHICKEN)
Compiled & edited by-DR RAJESH KUMAR SINGH,(LIVESTOCK AND POULTRY CONSULTANT) JAMSHEDPUR, JHARKHAND,INDIA 9431309542,rajeshsinghvet@gmail.com
Collection of blood specimens—–
Two different kind of blood specimens can be collected for infectious disease diagnosis (which makes exception of blood count and biochemistry):
• the whole blood for pathogen identification,
• the serum for serological diagnosis.
Blood samples are collected by venal puncture on live animal, as cleanly as possible, using sterile equipment, and with respect for the welfare of the animal:
• Use new needle and syringe for each animal,
• Dispose needles properly,
• Clean the puncture area with 70% alcohol,
• Handle animals calmly and using appropriate restraint,
• Although most techniques do not require large amount of blood it is preferable to collect at least 2.5mL of blood, but ideally 10mL in large animals, and 4-6mL in small animals,
• Identify the sample with a permanent method and fill the sampling form at the time of sampling.
Where the samples is taken from depends on the type of animal and fields conditions. When blood is taken from small veins it is better to use a syringe instead of a vacutainer to prevent the vein collapsing. Possible sites include:
• Jugular veins (large animals),
• Caudal vein (cattle),
• Brachial, brachiocephalic, femoral, tarsal veins,
• Cranial Vena cava in pigs,
• Mammary veins,
• Ear veins in pigs: 20 gauge needle,
• Saphenous veins, retro-orbital veins, intracardial punction in rodents, small vertebrates, use a 23 gauge needle,
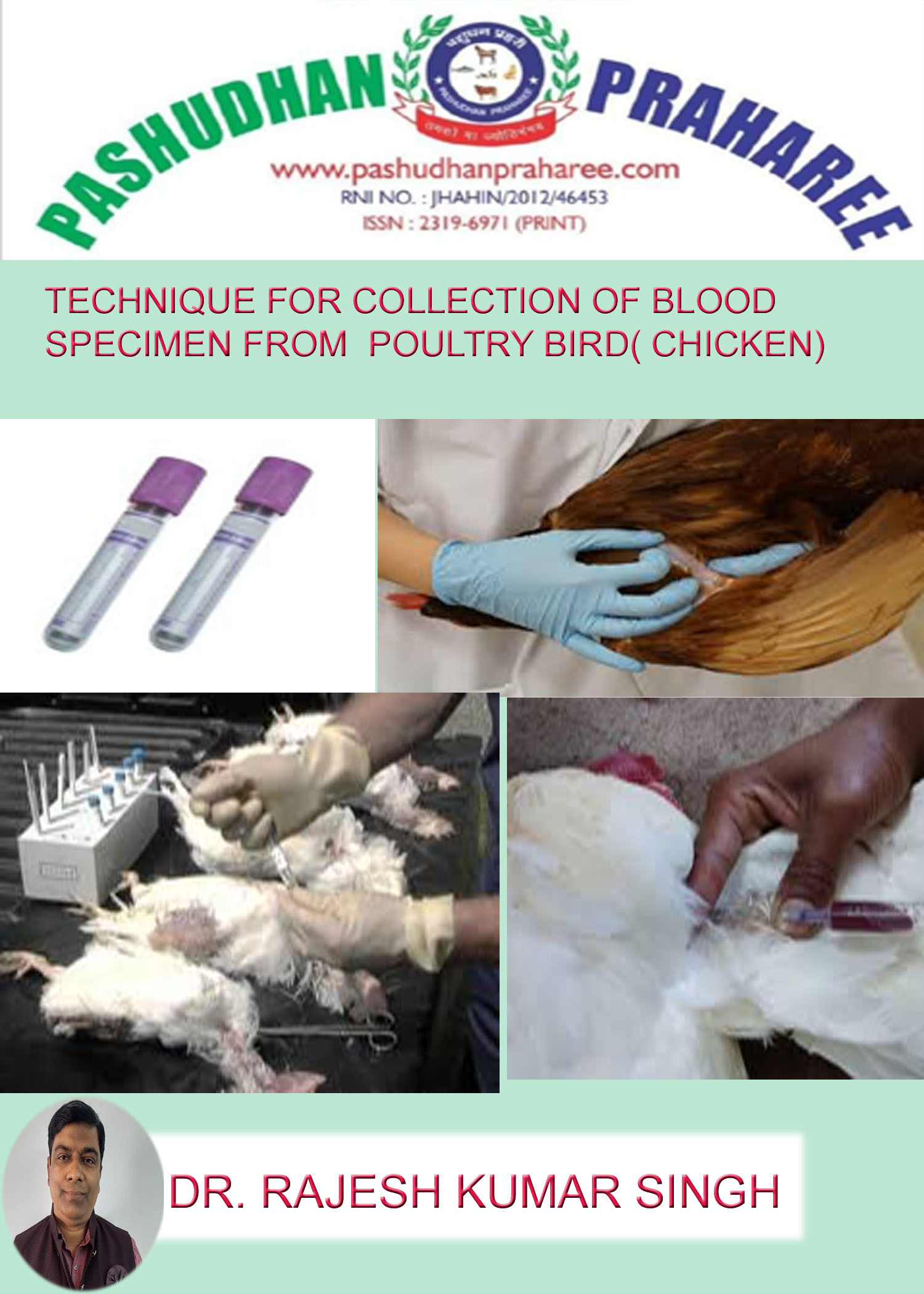
• Wings (brachial) in chicken, use 20 to 23 gauge needle.
Processing and conservation of samples depend on the type of examination to be undertaken.
Whole blood is prone to hemolysis, which is caused by red cells and interferes with a number of diagnostic tests. To help prevent hemolysis:
• Preferentially use a vacutainer instead of a syringe, if you use a syringe fill it with negative pressure,
• If you have to transfer the blood from a syringe to a sample container, remove the needle first and transfer slowly and smoothly,
• Do not shake the blood,
• Do not freeze whole blood.
Whole blood samples
For many infectious diseases, the agent can be identified from blood, by detection of antigens or culture and isolation of the agent, or demonstration of DNA.
In order to do such examinations the blood needs to be stored properly , with an anticoagulant. Different anticoagulants are available in commercial tubes such as: EDTA (purple tubes) or Heparin (green tubes) which are commonly used for bacteriology or virology, they have specific proprieties and it is better to refer to disease factsheet and/or laboratory to know which is the best anticoagulant to use. However EDTA is often recommended.
Blood is carefully transfer from the vacutainer or the syringe to the tube and gently invert 2-3 times to thoroughly mix the anticoagulant with the blood.
For virology antibiotics may be added, it is better to contact the laboratory first to have technical recommendations.. The usual method is to add penicillin and streptomycin to give final concentrations of 200 units of penicillin and 200 mg of streptomycin per ml of blood.
The blood container should be firmly capped and taped to prevent leakage.
Whole blood samples should be sent to the laboratory in an insulated container with ice at 4°C. They should not be frozen. Blood samples for virology must be sent to the laboratory within 24 to 72 hours depending on virus survivability.
Blood for serum samples
Serum samples are used for serological analysis. A positive serological reaction does not necessary means that the animal is clinically infected: vaccination, post-infection immunity, persistence of maternal antibodies in young animals, and lack of specificity of the test, are common cause of “false” positive.
Therefore, when possible it is always better to provide either paired samples from the same animal with 2-3 weeks between sampling or from different animals at different stage of the disease.
However in an outbreak of an exotic disease, a single positive reaction should be considered highly significant.
To collect serum from the blood it is important to let the tubes stand at ambient temperature for 1-2 hours in an upright position to let the clot begin to contract. The clot can be removed using a sterile rod after tubes are placed at 4°C for 12 hours. The serum can then be removed with a pipette or decanted in fresh tubes but if necessary the sample can centrifuged at 1-3000g for 10 minutes and the clear serum removed . It is possible to freeze serum at -20°C or even colder to preserve samples for later analysis. Plasma samples can also be used for serological diagnosis (blood should be cooled in an ice bath and centrifuged as soon as possible; then the plasma should be separated immediately after centrifuging).
If a bacterial disease is suspected, it is often useful to keep and submit the clot to the laboratory for bacterial culture.
Hemolysis can interfere with many serological diagnosis tests.
Blood is collected from chickens for two purposes:
1. To obtain serum which will be tested for Newcastle disease virus antibodies, no anticoagulant is required and the blood is allowed to clot. The levels of antibody detected in individual birds and flocks give an indication of the response to a vaccination. It also indicates whether birds have been challenged by field strains of Newcastle disease virus.
2. To obtain red blood cells, the blood is collected into anticoagulant. The cells are washed and used to test for the presence of virus in the haemagglutination test. They are also used in the haemagglutination inhibition test for the presence of antibodies.
It is important that those who bleed chickens use a quick and effective technique. This will develop with practice and by applying the following advice.
1- Handle the chickens gently.
2- Collect the blood samples quickly.
3- Take care not to damage the vein. Damaged veins will result in haematoma being formed.
4- Minimize the loss of blood. This minimizes trauma to the chickens and stress to their owners. The owners are then more likely to cooperate by supplying chickens for the collection of blood samples in the future.
Wing vein bleeding:
Materials
1- 2.5 mL syringes
2- 25 gauge needles for small chickens
3- 23 gauge needles for larger chickens
4- Cotton wool
5- 70 percent alcohol solution
6- Labels or marking pen to label each syringe
Method
1. Ask an assistant to hold the chicken horizontally on its back. The assistant uses one hand to hold the legs and places the other hand under the back to support the chicken.
2. Pull a wing of the chicken out towards you.
3. Note the wing vein, clearly visible running between the biceps and the triceps muscles. The wing vein forms a V (bifurcates). Note the tendon of the pronator muscle that runs across the V.
4. Pluck away any small feathers that obscure the vein.
5. Disinfect the area around the bleeding site by swabbing with 70 percent alcohol.
6. Insert the needle under the tendon. Direct the needle into the wing vein in the direction of the flow of blood. Do not insert the needle too deeply. Keep clear of the ulnar nerve.
7. Once the tip of the needle is in the vein, gently pull the plunger of the syringe. Blood will flow into the syringe. If blood does not flow, release the plunger and make a very slight adjustment to reposition the end of the needle.
8. Be patient and use a gentle suction to withdraw the blood. Chicken veins collapse readily.
9. If a haematoma forms, try bleeding from the other wing.
10. After removing the needle, apply pressure to the vein for a few seconds to discourage further bleeding.
11. Ideally the needle should be removed into a needle disposal container and the cap place on the end of the syringe to prevent leakage of the serum. However in many places these containers are not available and the cap will be placed over the needle.
TAKE CARE: Do this very carefully to avoid a needle stick injury.
12. Pull the plunger back approximately 1 cm and place the syringe at an angle with the needle end up in a rack facilitate clotting.
Bleeding a chicken alone:
It is a method for bleeding chickens without an assistant. The method is a modification of the procedure described above and is written for a right-handed person. Left-handed people soon make their own modifications once they start bleeding chickens alone.
1. Sit on a chair. Turn the chicken on its back and place it on your thighs.
2. Arrange the chicken with its head away from you and place the right wing securely between your thighs.
3. Use your left elbow to secure the legs by holding them down onto your left thigh.
4. Place your left forearm across the chicken and use your left hand to spread out the left wing of the chicken.
5. Use your right hand to bleed the chicken as described above.
The brachial vein (wing vein) is usually the most convenient site to obtain blood samples of broilers, broiler breeders, layers and turkeys. Heart puncture can also be used, but it is usually used as a method for obtaining blood from small chickens (one to several days old).. Chickens can also be sent live to the lab for blood collection. It is also very convenient to collect blood samples at processing plant.
Obtain at least 2 ml of blood from each bird. Do not fill more than half of the capacity of the tube. After the collection, lay the tube down so that it is horizontal or nearly so. Leave it until the blood clots. After the clot is firm, the vial may be returned to a vertical position. An occasional sample may require a long time to clot. A fresh blood sample should never be refrigerated immediately after collection, as this will hinder the clotting process. Keep the tube with the blood sample at room temperature for the next few hours or in the incubator at 370 C if possible. You may also leave the tubes at room temperature over night.
Ship blood in a cooler at 40 C. Blood must reach the lab within 24-36 hours after the bleeding of the animals. If blood can not reach the lab in that time frame, separate the serum (the clear fluid) and send the serum to the lab (chilled or frozen). These samples will be appropriate for testing samples for specific antibodies and or antigens
Reference-on request