PRACTICAL ASPECTS OF Artificial Insemination( A.I.) IN DOGS : Semen Collection, Preservation and Artificial Insemination
Dr. Pankaj Sinha
Incharge, Govt.Pet Clinic,Deoghar, Jharkhand, India
Artificial insemination is an imitation of the natural act of mating. It involves instillation of semen removed from a male dog into the cranial vagina or uterus of a bitch during the most fertile time of her estrous cycle. It is most frequently indicated in bitches that have normal estrous cycles but have a history of failure to conceive after natural mating or inability to be bred naturally. Successful artificial insemination results in pregnancy.
Artificial insemination is an assisted reproductive technique that can be used to compensate for some causes of canine infertility. Many reasons may lead to the request for artificial insemination in dogs.
Prebreeding Concerns
Physical Examination
Ensuring that both the dam and sire are healthy is important. The general health of the dam and sire is assessed with a breeding soundness and prebreeding examination, respectively.Only healthy animals without heritable genetic defects should be considered for artificial insemination. Information concerning possible heritable diseases should be obtained by questioning the owner. A general physical examination of the dam should be performed before, not at the time of, insemination to identify any problems. Likewise, the sire’s semen quality should be ascertained well in advance of the insemination procedure. Poor-quality semen may result in an unsuccessful pregnancy or contribute to small litter sizes.
Even with a healthy dam and sire, insemination may not be successful. The experience of the person performing the procedure and the difficulty of the technique used may influence the outcome. Coordination of ovulation in the bitch, semen acquisition from the desired stud dog, and insemination during the most fertile period of estrus is the most critical aspect of artificial insemination. Therefore, the owner should be made aware of every factor of artificial insemination before proceeding, including the time commitment and financial cost of the procedure.
Estrus and Ovulation
The estrous cycle in bitches is divided into four stages: proestrus, estrus, diestrus (or metestrus), and anestrus. Artificial insemination is always performed during estrus because it is the stage when ovulation occurs. This is the most fertile time of the estrous cycle.1-3 Estrus can last from 3 to 21 days in bitches, but the average duration is 9 days.3,6 External signs and the behavior of the bitch during proestrus and estrus are related to hormonal changes.
During proestrus, follicle-stimulating hormone secreted by the pituitary gland induces the development and growth of ovarian follicles. The ovarian follicles secrete estrogen, and as estrogen levels increase, the vulva begins to swell and the uterus releases a sanguineous discharge. The swelling of the vulva is caused by swelling and proliferation of the vaginal mucosa. These changes can be seen via endoscopy as an increase in the height of the longitudinal vaginal folds. The vaginal epithelial cell type also changes in preparation for copulation; detection of this cell type on a vaginal smear indicates an increase in estrogen. The male dog becomes interested in the bitch as a result of pheromones that are secreted at this time. The bitch may seem interested in the male dog but is not receptive to mounting and intromission.
In early estrus, the ovarian follicles continue to grow and secrete estrogen. As estrus progresses, the estrogen levels begin to decline as the luteinized follicular cells begin to produce progesterone. In late estrus, declining estrogen levels and rising progesterone levels trigger the secretion of a high level of luteinizing hormone (LH) from the pituitary gland. Ovulation occurs 24 to 48 hours after this peak increase in LH. At this time, the vulva is turgid and less swollen. The uterine discharge becomes serosanguineous and decreases or completely stops. The predominant cell type of the vaginal mucosa changes again, the vaginal mucosa becomes less edematous, and the bitch becomes receptive to the dog.
Released oocytes cannot be fertilized immediately after ovulation, when they are at the beginning of the uterine tube. The most fertile days are 2 to 3 days after ovulation,3,6 when the oocytes have descended through most of the uterine tube and are ready and available for fertilization. Natural mating or artificial insemination can be instituted during this 2- to 3-day period.
After ovulation, the remaining follicular cells develop into corpora lutea (yellow bodies) that continue to secrete progesterone. This continued rise in progesterone signals the onset of diestrus. In diestrus, there is another change in the primary cell type of the vaginal mucosa and no vulvar discharge. Vaginal and vulvar swelling decrease, the vulva gradually decreases in size, and the bitch again becomes nonreceptive to the dog.
Methods of Ovulation Timing
Ovulation timing is the determination of the most fertile days of estrus. Ovulation timing is used as part of the artificial insemination procedure to ensure that ova are present in the uterine tube at the time of the semen injection. An advantage of ovulation timing is that it allows the time of whelping to be determined exactly, which is especially important when a cesarean section is needed.
Vaginal Cytology
Vaginal cytology is the laboratory test most commonly used to confirm proestrus and estrus. For this test, a microscope slide is prepared from vaginal smears made by inserting a sterile cotton swab through the vulva into the vagina, avoiding the vestibule. A speculum or finger may be used to guard the swab on entry into the vagina. The cells from the cranial vagina walls easily exfoliate upon swabbing. After the cells are collected, the swab is rolled onto a glass microscope slide. The slide is allowed to air dry and is then stained, usually with a Diff-Quik stain, although other stains can also be used.
In proestrus, vaginal epithelial cells change from small parabasal cells with large nuclei to larger intermediate cells and then to very large cornified cells. The parabasal cells have basophilic blue-staining cytoplasm. The nuclei are easily seen, and the amount of nuclear material is roughly equal to the amount of cytoplasm. Intermediate cells are larger than the parabasal cells and less basophilic. The nuclei are smaller in comparison to the cytoplasm, and the cell borders are still somewhat rounded. Cornified cells have small or nonexistent nuclei and are large and square. High numbers of intermediate and cornified epithelial cells, red and white blood cells, and bacteria are evident during proestrus.
In estrus, the number of cornified cells increases. At the time of ovulation, the primary epithelial cell type seen on vaginal cytology is the cornified epithelial cell. The microscopic visualization of primarily (usually 90%) superficial epithelial cells and abundant bacteria without white blood cells indicates late estrus and probably ovulation.
The accuracy of vaginal cytology depends on the experience of the person(s) performing the swab, preparing the slide, and reading the slide. Therefore, it may be beneficial to use additional methods of determining ovulation to confirm the results of vaginal cytology.
Vaginoscopy
The hormone-related changes in the vaginal mucosa that take place during proestrus and estrus can be easily seen with the proper equipment and are consistent enough to determine the time of ovulation.6 The vaginal mucosa can be examined using a flexible endoscope, a pediatric sigmoidoscope, or, in very small dogs, an otoscope. Rigid endoscopes (cystoscopes) are also available for this purpose and for the observation of transcervical insemination. Observation of the vaginal mucosa to determine ovulation timing is referred to as evaluation of vaginal mucosal crenulation.
The increase in estrogen during proestrus leads to vaginal mucosal edema. The mucosa appears swollen and smooth and makes visualization of the vaginal lumen difficult. As the estrogen levels decrease, so does the edema. This decrease in swelling leads to a wrinkling of the vaginal mucosa, called crenulation. Initial crenulation indicates the beginning of the LH peak as the estrogen levels drop. After ovulation, marked wrinkling is seen and the vaginal lumen appears open. The disadvantages of determining vaginal mucosal crenulation are the requirements of prior experience, additional equipment, and sedation.
Hormonal Assays
Progesterone Assays
Measurement of serum progesterone during the estrous cycle can also be used to determine ovulation. The use of serum progesterone levels to determine ovulation is based on a normal physiologic increase in progesterone from basal concentration levels (<1 ng/dl) to >2 ng/dl just before ovulation. At the time of ovulation, progesterone levels increase to >4 ng/dl; after ovulation, they are even higher (levels vary with number of follicles). Progesterone is secreted by the corpora lutea after ovulation and signals that the follicle has ruptured and released the egg (ovulation has occurred).
Progesterone levels are measured using a serum sample. The commercial laboratory usually performs a radioimmunoassay or chemiluminescence test to determine serum progesterone levels. It is important that the commercial laboratory measure canine progesterone levels because the assay is species specific. This testing is readily available to the practicing veterinarian; however, a disadvantage may be the turnaround time for obtaining results. Obtaining results over holidays and weekends also may be problematic. Choosing a laboratory that performs the assay daily, allows overnight delivery of samples, and is willing to phone, fax, or email results daily is recommended. Planning is important to ensure that the laboratory will be available when needed. Laboratories that perform this service may be readily found locally or online. Some offer chilled semen service along with progesterone assays.
In-house tests that measure canine progesterone are available. Most require a serum or plasma sample. All require refrigeration and have a shelf life of about 60 to 90 days. The manufacturer’s directions must be followed carefully for each test. Samples must be prepared properly because hemolysis may cause a false reading in some tests. Lipemia may also cause false readings on some tests; before these tests are used, the animal may need to be fasted. The tests usually come with standard positive and negative samples for comparison. Results are given as a range rather than a specific number value. The available tests are the PreMate (Camelot Farms, College Station, TX), K9-Proges-Check (Endocrine Technologies, Newark, CA), Status Pro (Synbiotics, San Diego), and Target Canine Ovulation Testing Kit (BioMetallics, Princeton, NJ).
Luteinizing Hormone Assays
The accuracy of determining ovulation timing can be improved by adding an in-house LH assay to the progesterone assay. The LH assay measures the peak LH level that occurs before ovulation. Canine LH assays are available at some reference laboratories but are expensive and not widely used clinically. However, two in-house assays — the K-9 Ovicheck (Endocrine Technologies) and the Status-LH (Synbiotics) — are available. The K-9 Ovicheck assay is a quantitative assay marketed for research professionals to monitor physiologic and pathologic conditions related to circulating LH. The in-house use of this test is limited by its availability and complexity (it requires a two-step incubation process) and the time needed to complete the assay (3 hours). The Status-LH assay is a semiquantitative ELISA that is more readily available and less time consuming. However, because this test is semiquantitative, the results are either positive or negative. The results turn from negative to positive with an increase in serum LH levels consistent with the LH surge. Daily testing with this kit is required, and because preovulatory peaks can occur, an in-house progesterone assay should also be used to ensure that the LH peak is associated with ovulation. Therefore, the major disadvantage of this assay is that it requires serial serum sampling and must be combined with the progesterone assay for accuracy. Ovulation occurs 2 to 3 days after the LH peak; at this point, the progesterone assay should be >4 ng/dl.
Semen Preparation
Fresh, chilled, or frozen semen may be used for artificial insemination. Semen is collected from male dogs using an artificial vagina and manual stimulation. Latex products should be avoided because latex has been reported to decrease sperm motility.7 Collected sperm should be analyzed for numbers, viability, motility, and morphology.4,5 The conception rate is best with fresh semen (80%), followed by chilled (60%) and frozen (50% to 60%), but may vary according to the insemination technique used and the skill of the operator. The conception rate also depends on the proper handling of the semen and the fertility of the bitch.
Semen Analysis
Semen analysis, consisting of a sperm count to determine sperm concentration, motility analysis to determine sperm viability, and morphology analysis to identify sperm abnormalities, is performed to determine semen quality. Sperm concentration varies; therefore, sperm counts are conducted using a 1/100 Unopette dilutor (Becton Dickinson) and a hemocytometer. The diluted semen sample is used to charge the hemocytometer, which is placed under the microscope. The sperm present in the middle square of the nine large squares of the hemocytometer are counted and the number multiplied by 1 million to give the number of sperm cells per milliliter. This is multiplied by the volume of the ejaculate to determine the total number of sperm per ejaculation. Sperm counts of >200 million are usually seen in the rested stud dog. Counts of at least 200 million motile sperm are required for reliable vaginal artificial insemination.
Viability is determined by the amount of sperm with forward motility. Therefore, motility is considered the most important parameter in measuring semen quality. Motility can be analyzed by placing a drop of undiluted semen on a slide and placing it under the microscope for examination at 200 to 400x magnification. At least 70% of the sperm should exhibit forward motility.
Sperm morphology is analyzed to determine the percentage of morphologically normal sperm in the semen. Abnormal morphologic changes are divided into primary and secondary abnormalities. Primary abnormalities occur in the testes during spermatogenesis. Examples of primary abnormalities are abnormal heads; double midpieces and/or tails; frayed, thickened, bent, kinked or ruptured midpieces; and coiled tails. Secondary abnormalities occur during transit through the epididymis, semen collection process, or preparation of the slide for evaluation. Examples of secondary abnormalities are detached normal heads, detached acrosomes, and bent tails.
Sperm morphology is evaluated by mixing a drop of semen with a drop of eosin-nigrosin stain. The mixture is carefully drawn slowly across the slide like a blood smear and allowed to air dry. Sperm morphology is observed at 1,000x magnification under oil immersion. Normal morphology should be >60% normal sperm that do not present with head, proximal droplet, or tail abnormalities. Primary and secondary defects should constitute <10% and <20%, respectively, of abnormalities seen. Morphologic abnormalities in more than 20% of sperm have been reported to lead to a decrease in litter size.
Fresh Semen
Fresh semen has a short life span without preservatives and an energy source. Fresh semen can be immediately instilled by artificial insemination methods if the female has already undergone ovulation timing.
Chilled Semen
Frozen or chilled semen can be used if the male dog is not at the same location as the bitch. To prepare chilled semen, fresh semen is centrifuged to make a pellet, which is resuspended in a commercial extender that serves as an energy source for the spermatozoa. The semen is slowly chilled for an hour to preserve the longevity of the sperm during transport. The method used for further preparation and packaging depends on the semen supplier. Chilled semen must be shipped overnight or as soon as possible (ideally in 24 hours) to the owner of the female dog that is to be bred. Testing to determine ovulation timing must be conducted before the semen is shipped.
Frozen Semen
Frozen semen is initially prepared in the same way as chilled semen. The extender for frozen semen contains glycerol to prevent damage to the sperm during the freezing procedure. After the semen has been chilled for an hour, it is placed in straws or pelleted and frozen using dry ice or liquid nitrogen, depending on the semen supplier. The frozen semen samples must be labeled with the stud dog’s name or other identification and the date of freezing. Semen prepared in this manner can be shipped frozen for use at a later date; therefore, ovulation timing is not necessarily performed before the semen is shipped. Frozen semen must be kept at -4°F (-20°C) until needed and must be used immediately after being thawed. The sperm have decreased viability after the freezing process and must be placed directly into the uterus. Therefore, frozen semen cannot be used with intravaginal insemination.
The first recorded and successful artificial insemination (AI) was performed in the dog in 1785 by Spallanzani, the first successful use of frozen canine semen was reported by Seager in 1969. However, only in the last two decades AI in the dog became a rather widespread and generally accepted breeding technique. It may be applied for medical reasons, to prevent infections, to overcome travel and quarantine restrictions and to avoid long, expensive and stressful travels with the animals. In conjunction with cryopreservation AI is especially indicated to build up stocks from excellent studs and for conservation of semen from rare breeds. Only semen meeting the quality criteria outlined in table 1 should be used for conservation. The same quality criteria apply when studs are examined for breeding soundness.
Semen collection
The most common method for semen collection in dogs is by manual dissemination, needing no further elaborated equipment. It is generally no problem in young and possibly hypersexual and in trained dogs; it may be problematic in experienced males used for natural breeding. In these cases the presence of a teaser bitch at the optimal stage of oestrus may be essential and efficient stimulation may only be obtained when the stud is allowed to mount. To our experience the preparation of bitches not in heat with methyl, a component of vaginal secretions of female dogs in oestrus, has no satisfying effect on male dogs not responding to manual stimulation. Semen collection should be performed in a quiet and non-distracting environment. Typically, canine ejaculates are collected as three fractions: a small pre-sperm fraction, the sperm rich fraction and a more voluminous post-sperm fraction mainly provided by the prostate. A lack of the milky sperm rich fraction may be due to hypo-/azoospermia, obstruction of seminiferous ducts, retrograde ejaculation or an irregular course of the ejaculation reflex due to insufficient stimulation. Retrograde ejaculation may be confirmed by the detection of numerous spermatozoa in urine sediments after mild centrifugation. First results suggest that in dogs alkaline phosphatase activities in the sperm rich fraction can be used as a marker to monitor depletion of epididymal sperm stores during semen collection, as this enzyme is highly expressed in the epididymal tail (Gobello et al., 2000). In the dog semen quality in general is not improved in a short termed second ejaculate; however, 70% more spermatozoa may be obtained compared to a single ejaculate (England, 1999). Alternatively, canine semen can be collected applying electroejaculation under general anesthesia, and pregnancies have been obtained after the use of cryopreserved epididymal sperm, even after postmortem extraction (Marks et al., 1994, Hori et al., 2004).
Semen preservation and dose for insemination
Except for transfer of native semen only the sperm rich fraction should be used for semen preservation.
Native Semen
Native semen may be transferred due to medical or behavioural reasons without any further handling. The ejaculate may be split, however, transfer should be performed as soon as possible after semen collection and evaluation, although fertilization rates of 70% have been observed with unextended canine semen after two days of storage at 4°C (Tsutsui, 2002).
Table 1. quality criteria for dog semen (Hoffmann, 2003)
| forward motility | > 75 % |
| pathomorphology | < 20 % |
| pH-value | 6.2-7.2 |
| total number of spermatozoa | > 0.3-1.0 x 106 (1) |
| volume | 0.5-2.0 (2) |
- depending on breed; e.g., Dachshund >0.3; Dobermann >1.0
2. Sperm rich fraction
Chilled Semen
The use of chilled semen requests the availability of the donor on a short call and semen transport within a short term. A number of semen extenders has been described; generally accepted is a tris-buffered solution containing 20% egg yolk, 12.5% fructose and an admixture of an antibiotic. The cooling down to about 5°C is over a period of 2 hours. Depending on the concentration of spermatozoa, the ratio (volume) of ejaculate to extender may vary between 1:3 to 1:5. Mild centrifugation might become necessary when a selective collection of the sperm rich fraction was not possible. The total dose is stored in an appropriate sealed vial. The whole sperm rich fraction may be extended and used as one dose. If the ejaculate has to be split, one dose should at least contain 100-200×106 spermatozoa (Linde-Forsberg, 1991). The fertilization capacity of chilled semen decreases progressively, and it may be difficult to estimate the time after which the use of a certain semen portion may become unpromising, as after 5 days of storage motility may be still high, when fertilization capacity has considerably decreased (Tsutsui et al., 2002).
Frozen Semen
Whereas pregnancies may be obtained with fresh semen of inferior quality, albeit with smaller litter sizes, insemination of frozen semen of poor quality is unpromising. Thus, raw material of adequate quality and appropriate preservation procedures are indispensable for acceptable results. There are various protocols for cryopreservation of canine semen. Basically the same extender as used for the preparation of chilled semen may be used. The concentration of the cryoprotectant glycerin is at 6%. In our clinic semen meeting the criteria given above is diluted and packed in 0.5 ml straws. Following cooling to 5°C over 2 hours they are left for 10 min at-140°C (vapor of liquid nitrogen). Final storage is at-196°C. It is an absolute requirement to test the quality of each individual batch by thawing (37°C; 20 seconds) and reexamining a frozen aliquot, as the suitability for freezing may vary considerably between different ejaculates of an individual stud. Loss in forward motility should not exceed 20%, pathomorphology may be increased up to 30% (Hoffmann 2003). For insemination, a number of straws corresponding to approximately 1.5×108 progressively motile spermatozoa is thawed and pooled.
BREEDING OPTIONS IN THE BITCH
The goal of responsible dog breeders is to produce healthy puppies that adhere to specific breed standards and that can fulfill the jobs they are bred to do. With the advances in artificial insemination techniques that have occurred in the last 15 years, breeders have the option of choosing stud dogs from all over the world. That makes choosing the best brood bitch invaluable to a breeder, and knowing the options of breeding the bitch can help one achieve the best outcome.
Fertility in the bitch peaks when she reaches middle age (4 – 5 years) and declines thereafter. This allows breeders enough time to follow the guidelines of their breed clubs for pre-breeding genetic screening before breeding a bitch for the first time. Many breed clubs recommend waiting until bitches are at least two years old of age so that OFA X-rays, eye certifications, and thyroid testing can be done before the bitch is bred. Other breeds have even more stringent testing such as DNA markers for a variety of diseases. It is up to reputable breeders to maintain the quality of their brood bitches. Once the prescribed genetic testing has been performed, breeders must decide which insemination technique will be the best for the bitch depending on the type of semen to be utilized.
The bitch should be examined by a veterinarian who will take a thorough history, check for vaginal strictures, and perform a complete physical exam. A Brucellosis test is recommended since this disease is a contagious, potentially zoonotic (spread to humans), life threatening bacterial disease that can be transmitted via breeding or by coming into contact with infected material such as aborted feti, contaminated uterine secretions, semen or urine.
Guarded, cranial vaginal cultures are recommended on any bitch with a history of fertility problems. The culture is usually obtained on day two to three after bleeding begins and is sent for isolation of aerobic/anaerobic bacteria and Mycoplasma organisms. Many bacterial organisms, and even Mycoplasma, can be normal in the vaginal tract of bitches, but they can also cause infertility if they overgrow the normal bacterial flora of the vagina and are not treated. The veterinarian will start the bitch on the appropriate antibiotic if necessary. For bitches that do not conceive and have grown Mycoplasma on vaginal culture, it is recommended that they undergo treatment with doxycycline for four to eight weeks in between their heat cycles.
OVULATION TIMING
Ovulation timing is of the utmost importance, especially if you are using any type of processed semen, such as fresh chilled or frozen. The hormone that induces ovulation in the bitch is luteinizing hormone (LH). Testing for LH can be done using an in-house kit, ICG Status-LH1. LH testing must be done daily to catch the LH surge since it may only last 12 – 24 hours. Even with LH testing, it is recommended to confirm ovulation with progesterone assays. Progesterone testing is readily available in most cities in the United States. Different types of progesterone assays are available. In-house kits identify ranges of progesterone, and must be run daily to counter the inaccuracy of the method.
Commercial laboratories either use radio immunoassay (RIA), chemiluminescence (CLI), or enzyme linked fluorescent assays (ELFA). Some practices have in-house numerical progesterone machines (i.e. miniVIDAS or immulite machine) and can run progesterone samples in about an hour. Each veterinarian will have his or her own protocol as to when to breed bitches based on the type of breeding and their experiences.
Progesterone begins its normal rise at the time of the LH surge that stimulates ovulation. Serum progesterone concentrations are about 2 ng/ml on the day of the LH surge, and 4 -10 ng/ml on the day of ovulation. With each different type of assay the actual numerical value may differ, so be careful in memorizing a “magic” number. The eggs of the bitch are not ready to be fertilized at the time of ovulation. They must continue to mature, and it takes about two days before they are ready to be fertilized. The optimal days for breeding will be determined based on the day of ovulation and the type of breeding/insemination chosen.
NATURAL BREEDING
The vast majority of canine litters are produced by natural matings. Natural breeding is best used for young dogs and bitches that are thought to have good fertility. There is nothing wrong with presenting the bitch to the stud dog at about the time of ovulation and breeding the dogs every other day while she will stand and the stud has interest. It is best to bring the female to the stud dog and have supervised breedings so no fighting or injury occur. In young or inexperienced males, assistance may be required. Semen is not generally evaluated with a natural breeding, so litter size can be used as a potential indicator of semen quality. It must be remembered that small litter size may be due to sub fertility of either the male or female or a combination of both, so small litter size is not a direct indicator of poor semen quality, but rather an indicator that further workup of both male and female are required. With breeds that may require a Caesarian section, it is very important to determine the day of ovulation to help determine the timing of the surgery.
VAGINAL ARTIFICIAL INSEMINATION
The utilization of artificial insemination (AI) has been dramatically increasing over the past few years. Fresh AI offers the advantage of being able to evaluate the semen prior to insemination. The semen is collected with minimal prostatic fluid. An extender can be added if there is any decrease in sperm quality, or an alternative method of insemination may be chosen. An insemination pipette is gently inserted into the vagina with the bitches’ hindquarters being elevated for at least five minutes. There is some evidence that it may not be necessary to elevate the hindquarters after vaginal AI to obtain normal pregnancy rates and litter size. After injection of the semen using a syringe, light feathering of the vagina with a gloved finger is helpful in simulating the action of the copulatory lock. Vaginal AI is usually performed either on days 2 and 4 (or 1 and 3) after ovulation or on day 4 and 6 (or 3 and 5) after the LH surge.
TRANSCERVICAL INSEMINATION (TCI)
Transcervical inseminations are normally performed with an endoscope to visualize the cervix. An alternative method of TCI uses a blind catheterization technique (Scandinavian catheter) with a rigid vaginal catheter. Either method of TCI requires specialized equipment and training. With endoscopic TCI, a camera on the end of the endoscope allows visualization of the cervix, to allow for passage of a catheter into the uterus, where semen is deposited directly. This increases the number of sperm that enter the uterus as opposed to the loss associated with deposition in the vagina. Use of TCI decreases the number of sperm needed to doses as low as 100 – 200 million normal, motile sperm. The uterus of the bitch can only hold about 2-3 milliliters (ml) of semen depending on the breed (1 – 1.5 ml in toy and small breeds). The semen must either be collected in fractions (pre-sperm, sperm and post-sperm fractions) saving only the sperm-rich fraction for insemination, or the semen must be concentrated by low speed centrifugation and then resuspended with extender.
TCI is normally performed on the unsedated, standing bitch. It is not as easy to perform on maiden bitches, toy breeds and some giant breeds due to either the size of the vaginal canal near the cervix or to the fact that the cervix may not relax adequately prior to whelping to allow passage of the catheter. Lack of visualization of the cervix may occur due to blood and secretions pooling at the front of the vagina. Additionally, the cervix may not be able to be moved to the proper angle to allow passage of the catheter. In rare cases, the vaginal canal of some giant breed bitches may be too long for the scope to reach the cervix.
However, in most cases, in competent hands TCI is a most useful tool.
TCI is usually used with fresh or fresh extended semen. The benefits are that a smaller breeding dose is required and conception rates should be higher than with vaginal AI, especially when breeding bitches or dogs with fertility problems. TCI can be used with frozen semen, but surgical insemination is generally still preferred. The disadvantages of TCI include increased cost of the procedure and the need for extensive training and practice for reliable results. TCI is usually performed two times; once on day 2 and 4 (or 3 and 5) post-ovulation, or if performed once, then it is on days 3 or 4 post-ovulation.
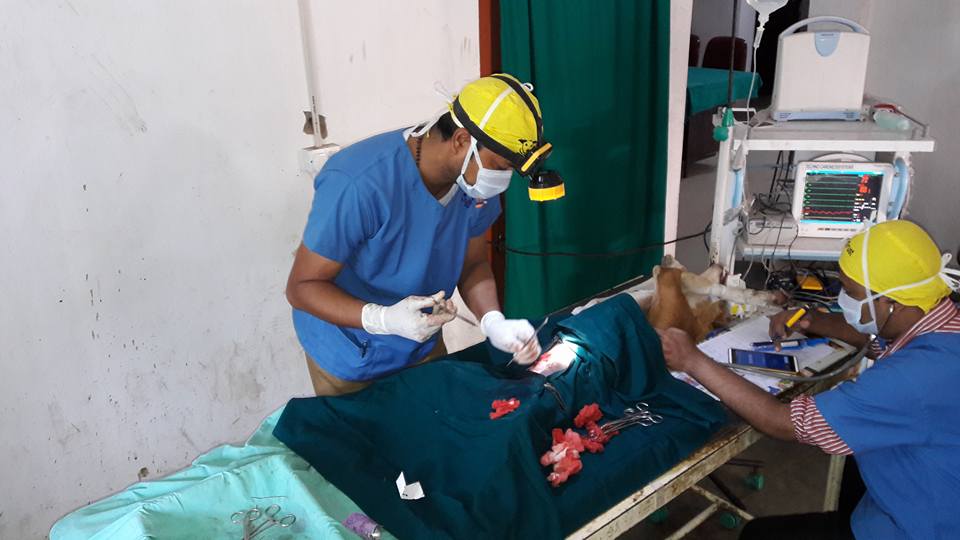
SURGICAL INSEMINATION (SI)
Surgical inseminations are a routine procedure. During the procedure, the bitch is anesthetized and her abdomen clipped and prepared for surgery. An abdominal incision is made (much like a spay) and the uterus is located. Semen is injected directly into the uterus using a needle or catheter. The entire procedure takes less than 30 minutes. One major advantage of the surgical insemination is that the uterus can be visualized, palpated, and evaluated for pathology. It is not uncommon to find cysts in the uterus that can be gently ruptured prior to insemination to try to improve fertility. Other common pathologic findings include adhesions or thickened uterine walls. With surgical
inseminations, the bitch is usually bred one time, 3 – 4 days after ovulation.
Surgical inseminations are useful with any type of semen where semen quality may be compromised, with frozen or fresh chilled breedings, or on females with fertility problems. Similar to transcervical inseminations, the volume inseminated must be small. Frozen semen is packaged such that a breeding dose is in a volume of 1.5 – 2 ml.
The advantages of surgical insemination are the ability to inspect the uterus and ovaries, delivery of the entire breeding dose directly into the uterus, and minimizing contamination during breeding. SI leads to the highest rate of conception in many cases. The disadvantages include cost, risk of anesthesia, risk of abdominal infection (peritonitis), and risk of incisional infection. With a single SI, the cost of the surgery plus semen shipment is often equal to or less than that required for 2 TCI.
MULTIPLE SIRE LITTERS
The AKC allows breeders to use more than one stud dog during a breeding.
This is especially useful if one is using semen from a sire that may be of questionable quality. Rather than losing the pregnancy due to failed conception, a second proven sire may be used to back up the sub-fertile sire. Generally speaking, the sub-fertile sire is inseminated first (on day 3 post-ovulation), using SI or TCI, and then the proven sire is inseminated 24 hours later (on day 4 post- ovulation), using TCI or vaginal AI (or natural breeding if a SI was not performed the day before). The use of 2 fertile sires will generally result in all the puppies being sired by only one of them, even if the semen from both sires is deposited at exactly the same time. DNA testing is required on the dam, both sires and all of the resulting puppies to determine parentage.
Insemination regime
As with natural mating, precise determination of the optimal time of insemination is less critical using native or chilled semen compared to cryopreserved semen due to the longevity of fresh canine spermatozoa in the female genital tract which is between 5-7 days, yielding a broad biological leeway for pregnancy even under suboptimal insemination management (Jeffcoate, Lindsay, 1989). Thus, using native or freshly provided chilled semen, a single insemination around the time of ovulation may be sufficient, although significantly higher pregnancy rates have been found with two inseminations (Linde-Forsberg et al., 1993). In contrast, the limited life-span of frozen-thawed spermatozoa in the female genital tract, which is estimated to be shorter than 12-24 hours (Concannon et al., 1989), requires a precise definition of the time of insemination in the individual bitch, which is about 2-5 days after ovulation due to a comparatively long postovulatory process of oocyte maturation. Repeating the AI after 24-48 hours may result in significantly higher pregnancy rates and litter sizes (Tsumagari et al., 2003) probably due to the occurrence of an extended ovulation and maturation period. Indications for the appropriate time of insemination may be obtained by observation of behavioural changes, vaginal cytology, vaginoscopy and determination of the electrical impedance. Repetitive examinations are necessary and the variability inherent to these approaches and the underlying parameters is high. It is therefore recommended to directly determine the underlying hormonal changes by applying a reliable quantitative assay for progesterone. Progesterone levels closely reflect the pre and postovulatory luteinization of granulosa cells with about 1-2 ng/ml during the LH-peak and about 5 ng/ml at ovulation. Termination of oocytes maturation is more difficult to assess due to the variability in the increase of progesterone levels with formation of the corpora lutea. However, progesterone levels between 10-12 and even up to 20 ng/ml are generally considered as optimal for insemination with cryopreserved semen (Linde-Forsberg et al., 1989).
Technique of semen transfer
Due to the anatomical structure of the vagina intrauterine insemination in the dog is more difficult than in other species and requires quite some experience. This is largely due to a dorsal median fold of tissue that extends caudally from the vaginal portion of the cervix. At vaginoscopy the caudal portion of the fold and the constrictions of the lateral and ventral vaginal walls give a distinct appearance, referred to as pseudocervix. The true vaginal portion of the cervix is cranial to the pseudocervix. This makes intrauterine cannulation difficult, also because the cervical canal is nearly perpendicular to the longitudinal axis of the vagina and the uterine body.
Currently four methods are used for semen transfer in dogs: deep intravaginal insemination, transcervical insemination using an rigid catheter (Norwegian Catheter) with transabdominal fixation of the cervix, transcervical endoscope-aided insemination and intrauterine insemination using a laparoscopic approach. Freshly ejaculated spermatozoa and spermatozoa from chilled semen may readily penetrate the cervix and reach the site of fertilization in the oviduct. Hence deep intravaginal insemination with this type of semen is common practice. As a substitute of the vaginal plug by the erected bulbus glandis it has been generally recommended to elevate the rear end of the bitch at a 45° to 60° angle for 5-20 minutes after AI. However, pregnancy rates and litter size was not different comparing elevation times of 1 and 10 min (Pinto et al., 1998). In respect to cryopreserved semen it was widely accepted that semen deposition must be intrauterine for optimal results. However, data from literature are contradictory as in some studies no significant difference was found between precervical and transcervical insemination using frozen thawed semen (Rota et al., 1999). Yet up to now the statistical basis is very weak not allowing any definite conclusions (see below). Hence we still recommend intrauterine semen deposition using the transcervical approach since the laparoscopic approach is not accepted by many pet owners, which consider this method as unethical and too risky for the bitch. Yet it must be anticipated that the transcervical approach may be difficult in maiden dogs and in those cases with a high discharge of cervical mucus, blinding the optical system. In these cases the semen should be placed as close as possible to the orificium externum of the cervix.
Methods of Artificial Insemination
Two methods of artificial insemination are described in the literature: intravaginal and intrauterine. The intravaginal method involves instillation of semen into the vagina using a syringe and a long plastic (insemination) catheter. Insemination catheters for species other than dogs (e.g., goats, cows, horses) may be used. The catheter is introduced into the vulva and advanced as far as possible into the vagina. When the catheter cannot be advanced any further, a syringe containing the semen is attached. The bitch is held at a 45° angle with her hind feet in the air while the semen is injected. The syringe is removed, and the bitch remains with her hind end elevated for 15 to 20 minutes to allow gravity to assist sperm transportation to the uterus. A gloved finger may be inserted into the vagina to gently stroke (feather) the vaginal walls to try to stimulate vaginal contractions to assist in moving the sperm cranially toward the cervix. This procedure requires little experience and can be used for multiple serial inseminations daily or every other day. The main disadvantage is that frozen semen cannot be used for this procedure.
Transcervical insemination involves instillation of semen into the uterus through the cervix. There are two methods described for transcervical insemination. The Scandinavian or Norwegian approach requires the use of a specialized metal catheter with a nylon sheath. The special catheter and sheath are introduced through the vulva into the cervix, which has been located by abdominal palpation. When the operator is sure that the catheter and sheath are in the cervix, the sheath is removed. The other transcervical method, known as the New Zealand approach, involves mild sedation and a rigid endoscope (cystocope) with a special port that allows visualization of the cervix and placement of an 8-Fr polypropylene catheter into the cervix. Using either transcervical technique, the semen is injected via syringe into the uterus after placement of the catheter. The bitch is then handled similarly to the intravaginal approach. The advantage of the transcervical approach is that because the semen is placed into the uterus, fresh, chilled, or frozen semen can be used. It is also safe enough to be used daily or every other day because only mild sedation may be required. The main disadvantage of transcervical insemination is that both procedures require skill and special equipment.
Surgical intrauterine instillation of semen involves direct injection into the uterus by surgical laparotomy. This procedure requires general anesthesia, a small abdominal incision to exteriorize the uterus, and incision into the uterus to instill the fresh, chilled, or frozen semen. Its major advantage is that it allows the operator to directly observe the injection of the semen into the uterus. However, it requires anesthesia and surgery. Therefore, it is usually only performed once because repeated surgical procedures and anesthesia place the bitch and puppies at risk. Furthermore, this procedure may be banned in some countries as inhumane.
Role of the Technician
As an assistant to the veterinarian, the technician needs to know what tests may be conducted and which insemination procedure will be used as well as the supplies and equipment needed for each. In some cases, the technician is involved with the prebreeding or breeding soundness examination. The technician may also have the responsibility of preparing and possibly reading slides for vaginal cytology, collecting samples and conducting in-house LH and progesterone assays, or collecting and mailing samples for commercial laboratory assays for progesterone and receiving the results. The technician may also be responsible for keeping up-to-date on what laboratory tests are available and which are preferred based on previous clinic experience.
Pregnancy rates and litter sizes
Success of AI in dogs depends on various parameters such as type and quality of semen used, sperm dosage, the method and site of semen deposition, insemination regime and breed.
Provided semen of adequate quality is used, success of AI using native or chilled semen is equal to that after natural mating (Pinto et al., 1999).
Several studies are available reporting pregnancy rates and litter sizes after AI with frozen-thawed semen in bitches using intravaginal and/or intrauterine insemination. However, results vary substantially obviously due to the high variability of the conditions for insemination. However, in a relatively large comprehensive study using semen of defined quality, pregnancy rates/liter size after a single insemination per cycle were 84.3%/5.0 ± 3.0 puppies after intrauterine semen deposition, but only 34.8% / 2.5 ± 1.3 puppies after intravaginal insemination. These data show that–provided a good method of cryopreservation and intrauterine semen deposition are applied–results with AI using frozen-thawed semen may be comparable to those obtained with natural mating. In those studies yielding no difference between intravaginal vs. intrauterine insemination, the benefit of intrauterine insemination may be overridden by higher doses of spermatozoa or by higher numbers of AI’s per cycle. Accordingly, in the above mentioned study whelping rate/litter size after intravaginal deposition of frozen-thawed semen increased to 63.9%/5.1 ± 3.1 when three inseminations per cycle were applied (Linde-Forsberg et al., 1999).
Conclusions
By now AI in the dog must be considered a well established breeding technique with the use of frozen semen gaining constantly more importance. Techniques for semen preservation, the quality assessment of semen and the dosages used are fairly standardized. Appropriate semen quality and AI management provided, results with native, chilled and even frozen-thawed semen may be comparable to natural mating. It is predicted that the use of AI will continue to rise in dogs with more AI centers becoming available, increasing acceptance by breeders associations and the abolition of import restrictions.
Coordination is key to increasing the probability of obtaining a successful pregnancy through artificial insemination. Although time-consuming, well-coordinated efforts are more likely to be rewarded with success. Without thorough planning, artificial insemination may be a frustrating exercise. Preexamination of the proposed dam and sire is needed to determine infectious, metabolic, or genetic defects that could lead to potential spread of infectious disease, failure to conceive, or early termination of pregnancy. Ovulation timing should be used to determine when the bitch is most fertile, especially when shipping chilled semen or when using frozen semen, if only one insemination is possible. Knowledge of the semen preparation used is important in order to determine the appropriate insemination method.
References
https://www.vin.com/apputil/content/defaultadv1.aspx?pId=11181&catId=30095&id=3852300
https://www.vetfolio.com/learn/article/canine-artificial-insemination
George F. Seier, Jr. D.V.M.