FLUID THERAPY PROCEDURE IN PETS
COMPILED BY-DR. AMIT BHARDWAJ,CANINE VET,PUNE
Fluid therapy, as the name suggests, is a procedure that helps replenish lost fluids and maintain electrolyte balance in critically ill animals. It’s considered one of the most common therapies used in animal clinics and hospitals today. However, it’s not a one-size-fits-all type of therapy and has to be tailored to fit the needs of the individual patient.
Fluid therapy is one of the most common therapies provided in small animal medicine. Patients are given fluids for many reasons, and the number of available fluids is growing. Knowing why fluids are ordered, the goals and limitations of fluid therapy, and how fluids are chosen is a key competency for veterinary technicians.
What Are the Different Types of Fluids?
There are different types of fluids used in fluid therapy. The most common ones are Normal Saline, Lactated Ringer’s Solution, and Acetated Ringer’s. Depending on the needs of your pooch, your veterinarian may give them one of the following:
Crystalloid Fluids
Crystalloid fluids are solutions of mineral salts combined with other small, water-soluble molecules. They’re typically the ones used for fluid replacement because of how effective they are in correcting improper electrolyte balance and preventing dehydration.
Examples of crystalloid fluids include Lactated Ringer’s Solution, 5% Dextrose in water, 10% Dextrose in water, and 3% NaCl Solution.
Synthetic Colloid Fluids
Unlike crystalloid fluids, synthetic colloid fluids have large molecules. Their purpose is to provide fluid support to maintain proper oncotic pressure—the force that helps fluids move across the capillary walls.
Examples of synthetic colloids include hydroxyethyl starch solution and dextrans,
Natural Colloid Fluids
Natural colloids are composed of blood products, like red blood cells, plasma, and albumin. Because of their components, these fluids have the capacity to effectively carry oxygen throughout the body, which is especially helpful in preventing or treating hypoxia—a condition where oxygen doesn’t reach the tissues.
Fluid therapy is used in the clinical setting for many reasons, including replacement of hydration deficits, to maintain normal hydration, replace lost blood volume, correction of electrolyte imbalances, treating shock, and in order to infuse intravenous medications. It is important for the veterinary technician to have knowledge of the types of fluids available, appropriate routes and rates of administration, and how to assess the patient’s hydration status.
There are two main types of fluids veterinarians will use in practice, crystalloids and colloids. As compared with crystalloids, colloids more effectively restore intravascular volume with smaller volumes, notably when treating hypovolemia and hypoproteinemia. Colloids are useful in volume support and resuscitation because they do not cross a normal capillary wall from the intravascular to the interstitial space as easily as crystalloids, prolonging their effect on intravascular volume resuscitation3.
Colloids are classified as either natural or synthetic. Natural colloids contain naturally produced proteins such as albumin. Albumin can be administered with blood products (frozen plasma, fresh frozen plasma and whole blood) or albumin replacement solutions, such as lypholized canine albumin or 25% human albumin. Plasma products have an albumin concentration and colloid osmotic pressure (COP) equal to plasma (18–24 mmHg) and therefore do not significantly alter the recipient’s COP. Conversely, 25% human albumin has a COP of 200 mmHg and will significantly affect the patient’s COP.9 Both acute and delayed reactions have been reported following human albumin administration requiring close monitoring for up to several weeks post transfusion therapy.
A colloid solution contains negatively charged large molecular weight particles that are osmotically active, drawing sodium around their core structures. Wherever sodium is, water follows. By drawing sodium around the particle, water is thus held within the vascular space. Colloids replace intravascular fluid deficits only. Therefore, colloids are always administered along with crystalloids, to restore both intravascular and interstitial fluid volume. Examples of artificial colloids include Hetastarch, Vetstarch, Pentastarch, and Voluven. Whenever a colloid is administered along with a crystalloid, calculated crystalloid fluid requirements should be decreased by 25% – 50%, to avoid intravascular volume overload. Natural colloid solutions include whole blood, packed red blood cells, and plasma. Fresh whole blood is indicated when loss of both red blood cells and plasma has occurred. The Rule of Ones states that one ml of fresh blood infused per one-pound body weight will increase the patient’s packed cell volume by one per cent, provided that no ongoing losses are present. Packed red blood cells can be administered when anemia is present in sufficient quantity to cause clinical signs of anemia, including lethargy, inappetance, tachycardia and tachypnea. Fresh frozen plasma can be administered at 10 – 20 ml/kg/day to replenish clotting factors and provide antiprotease activity during inflammatory conditions. Fresh frozen plasma can be used to replace small amounts of albumin, in cases of hypoalbuminemia, however, is not efficient as administering purified concentrated canine-specific (when available) or 25% human albumin. Frozen or fresh frozen plasma (20 ml/kg IV) needs to be infused for every 0.5 g/dL increase in plasma albumin, provided that no ongoing losses are present. The goal of albumin administration is to raise the patient’s serum albumin to 2.0 g/dL, then provide the remainder of colloidal support with synthetic colloids. Hydroxyethyl starch is a polymer of amylopectin suspended in a lactated ringer’s solution. The average molecular weight of Hetastarch is 69,000 Daltons. Larger particles are broken down by serum amylase, and last in circulation for approximately 36 hours. Because Hetastarch can bind with von Willebrand’s factor, mild prolongation of a patient’s APTT and ACT may be observed, but do not contribute to or cause clinical bleeding. Hetastarch should be administered in incremental boluses of 5 – 10 ml/kg in dogs, and 5 ml/kg in cats. Because rapid administration of hetastarch can cause histamine release and vomiting in cats, the bolus should be administered slowly over a period of 15 – 20 minutes. Some authors recommend that the total daily dose of hetastarch should not exceed 20 – 30 ml/kg/day. Following the administration of hetastarch boluses, it should be administered as a constant rate infusion (20 – 30 ml/kg/day IV) until the patient is able to maintain its albumin and colloidal support on its own. Concentrated human albumin and concentrated canine albumin solutions are now available for use in veterinary patients. Both immediate and delayed rare Type 3 hypersensitivity reactions have been documented in healthy and hypoalbuminemic dogs following administration of concentrated human albumin. Reactions that occurred that include fever, vomiting, acute anaphylaxis, urticaria, angioneurotic edema, and delayed vasculitis, polyarthopathies, glomerulonephritis and death in both healthy and critically ill animals. Although there are studies which have demonstrated adverse reactions and the development of anti-human albumin antibodies after concentrated human albumin infusion in dogs, there also have been studies which have documented improved clinical outcome when concentrated human albumin was infused into critically ill animals that were refractory to other more mainstream therapies, including pressors, synthetic colloids, and fresh frozen plasma transfusions. Concentrated 25% human (2 ml/kg IV in dogs over 4 hours; pre-treat with 1 mg/kg diphenhydramine IV). should be considered in any patient with refractory hypoalbuminemia (< 2.0 g/dL) or hypotension unresponsive to other synthetic colloids, pressors, and inotropes. The perceived benefits of albumin infusion and risks of not infusing albumin must be weighed against the potential risks of its administration. Clients must be aware of the potential risks of complications.
What is Fluid Therapy?
Fluid therapy is the act of replenishing a canine with adequate fluids when they have been depleted due to disease or trauma. Fluid therapy can be administered to a dog intravenously (through the vein), subcutaneous (under the skin), intraosseous (through bone marrow), or intraperitoneal (through the abdominal wall).
There are three different types of fluids that are used in canine fluid therapy:
- Fluids containing isotonic crystalloids match the same solute concentration of blood and therefore will mimic the osmotic pressure.
- Colloids supply oncotic pressure, found in both natural and synthetic norms.
- Hypertonic saline, which is a fluid therapy element that creates a high osmotic pressure within the vascular space.
Body Water Compartments
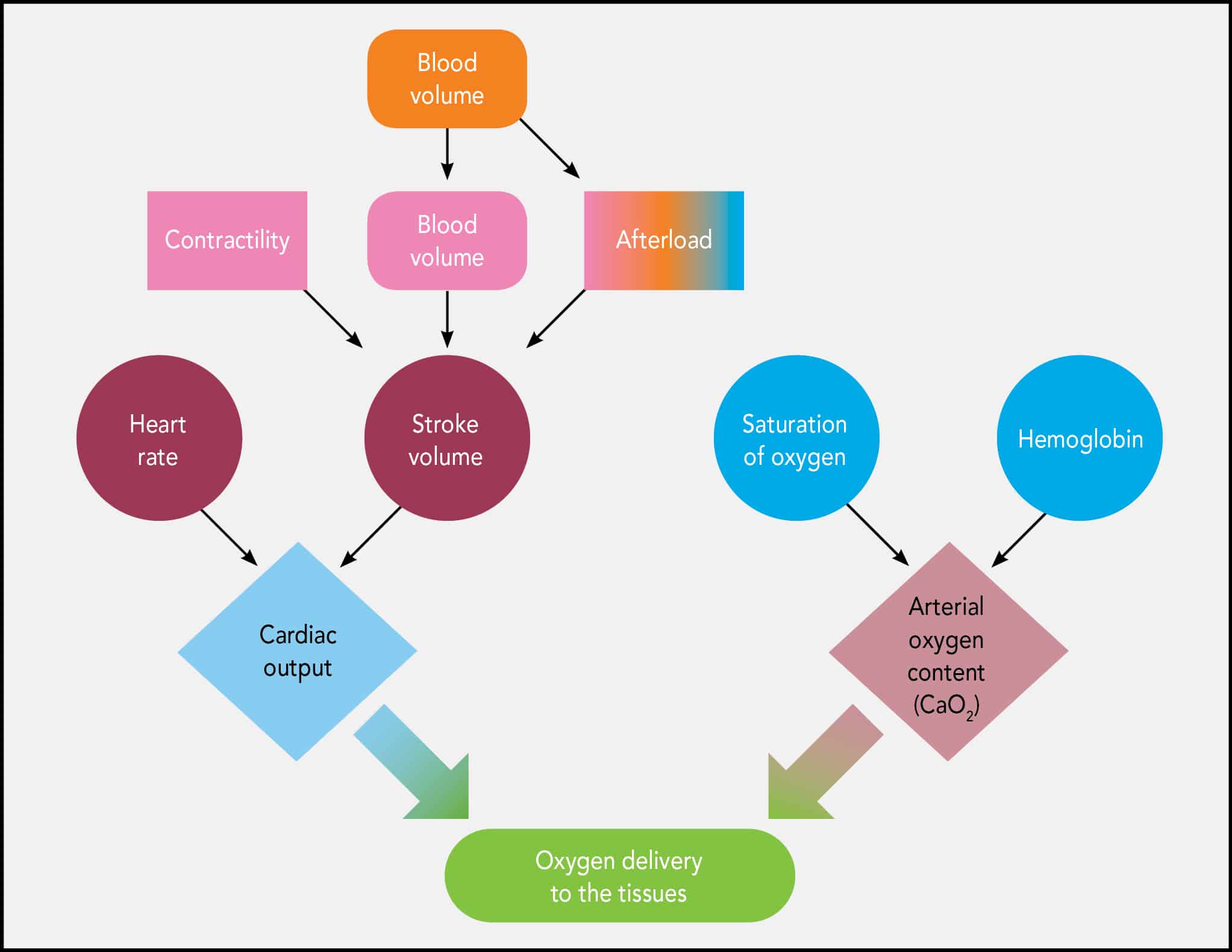
To understand fluid therapy and its applications, one must first understand the distribution of fluid and water in the body (FIGURE 1). Total body water (TBW) comprises approximately 60% of a patient’s body weight.1 Approximately 67% of TBW is found inside the body’s cells and is referred to as intracellular fluid (ICF). The remaining 33% of TBW is the extracellular fluid (ECF), which is further divided as follows:
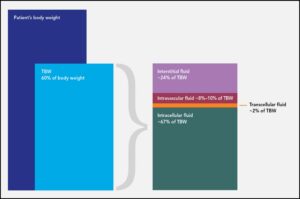
FIGURE 1. Fluid compartments in the body. Total body water (TBW) is 60% of a patient’s body weight and can be thought of as separated into distinct compartments, as represented here.
- Interstitial fluid, which bathes cells and tissues (~24% of TBW)
- Plasma, the liquid portion of blood, which constitutes most of intravascular volume (~8%–10% of TBW)
- Transcellular fluid, which comprises synovial joint fluid, cerebrospinal fluid, bile, and the fluid in the linings of the peritoneal cavity, pericardium, and pleural space (~2% of TBW)
A helpful rule of thumb to simplify the distribution of fluids in the body is the 60:40:20 rule: 60% of a patient’s body weight is water, 40% of body weight is ICF, and 20% of body weight is ECF.
The body is considered a closed system, meaning that any fluid lost must come from one of the compartments listed above. In the case of hemorrhage, for example, fluid is lost from the intravascular space (i.e., plasma) but also from the ICF in the cells lost (e.g., red blood cells, white blood cells). In addition to losses, fluid can and does move between compartments in a dynamic and ever-changing fashion. When providing fluid support to patients, technicians must keep in mind which compartment needs to be replenished or what derangement needs to be corrected. This knowledge helps guide both fluid choice and the method used to administer fluid therapy.
Reasons for Fluid Therapy
Veterinary professionals provide fluid therapy to patients for many reasons, including correction of dehydration, expansion and support of intravascular volume, correction of electrolyte disturbances, and encouragement of appropriate redistribution of fluids that may be in the wrong compartment (e.g., peritoneal effusion).
The first step in determining whether a patient needs fluid therapy is a full physical examination, including collection of a complete history. The veterinary staff must assess whether the patient is perfusing its tissues well, check for dehydration, and evaluate losses from any of the fluid compartments.
Inadequate Perfusion
Patients that cannot adequately perfuse their tissues require immediate intervention with fluid therapy to restore perfusion and correct shock. Shock is defined as the critical imbalance between the delivery of oxygen and nutrients (carried by blood) to tissues and the tissues’ demand for these components. If allowed to persist, this imbalance can lead to acute decompensation and death. Restoring perfusion and, subsequently, oxygen and nutrient delivery to tissues is crucial to survival in these patients.
BOX 1 Clinical Signs of Shock
- Vasoconstriction
- Pale mucous membranes
- Prolonged capillary refill time
- Peripheral temperature < core temperature
- Reduced urine output
- Decreased mentation
- Tachycardia (cats may present with bradycardia)
- Hypotension (poor pulse quality)
- Reduced oxygen saturation (low SpO2)
- Lactate >2 mmol/L
- Metabolic acidosis
Shock is a life-threatening emergency and must be recognized and treated immediately on presentation. Patients may present with several clinical signs (BOX 1), and owners may report a history of recent fluid loss, such as intractable vomiting, severe diarrhea, or hemorrhage. Once shock is recognized, access to the intravascular compartment must be achieved and fluid resuscitation initiated as soon as possible (see Ways to Provide Fluid Therapy), with the goal of restoring intravascular volume and flow, thus improving perfusion and delivery of oxygen and nutrients to starving tissues (FIGURE 2).
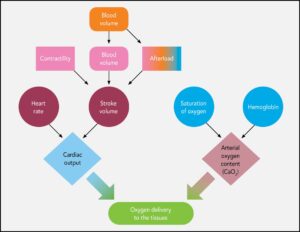
FIGURE 2. Oxygen delivery to the tissues (DO2), which is crucial for maintaining cellular metabolism and preventing cellular death, depends on many factors.
Oxygen delivery to the tissues (DO2) depends on cardiac output and arterial oxygen content. Cardiac output is the product of stroke volume and heart rate. Stroke volume is defined as the amount of blood ejected from the left ventricle during systole and is a product of preload (the amount of blood entering the heart), afterload (the amount of resistance in the vasculature to the flow of blood from the heart), and contractility (the heart’s ability to contract). Once perfusion and, by extension, DO2 is restored, homeostasis can be reestablished and the shock state will be remedied. Correction of perfusion deficits is demonstrated by normalization of the forward perfusion parameters, listed in BOX 2.
BOX 2 Forward Perfusion Parameters
- Heart rate
- Pulse quality
- Respiratory rate
- Mucous membrane color
- Capillary refill time
- Mentation
- Temperature and color of digits
Dehydration
Loss of fluid from the intracellular and interstitial compartments leads to dehydration. If severe, dehydration can be detected in derangements in forward perfusion parameters1 as well as by the tests listed below. Any patient determined to be more than 10% dehydrated is considered severely dehydrated4 and requires immediate fluid resuscitation and careful monitoring.5 Dehydration must not be confused with hypovolemia: dehydration describes a water deficit in the interstitial and intracellular compartments, whereas hypovolemia describes a loss of fluid in the intravascular space.
Hydration status can be assessed using several simple tests. One of the easiest to perform is a skin tent test to check the turgor, or moisture level, of the skin. To perform this test, the skin over the thorax or lumbar region is pulled away from the back. In a well-hydrated animal, the skin immediately returns to its normal resting position. If the tent formed remains standing, it can be an indication of dehydration.1,5 When performing this test, veterinary technicians can often appreciate a “tacky” or “sticky” feeling in the underlying tissue, which is further evidence of dehydration. The skin tent test can be confounded by both emaciation (decreased turgor even if euhydrated) and obesity (increased turgor in the face of dehydration) and must be considered in relation to other parameters and physical examination findings. Age is another factor to consider: loss of skin turgor progresses with increasing age, and neonates exhibit very little skin tenting even when dehydrated.
Another way to check for dehydration is to feel for moistness on the mucous membranes. This is most easily accomplished by sliding a finger along a patient’s gum line or inside the cheeks. If the membranes themselves are dry or sticky, it may indicate dehydration. In the case of vomiting animals, it is necessary to differentiate excess saliva in the mouth from mucous membrane moisture.
In patients with normal kidney function, oliguria can indicate dehydration, and the small amount of urine produced will likely be concentrated (urine specific gravity [USG] >1.030).5 Other laboratory parameters that change with dehydration include packed cell volume and total protein (PCV/TP) levels, which demonstrate hemoconcentration (high PCV) and hyperproteinemia (high TP) in dehydrated patients5 due to the loss of the fluid portion of the blood as the body tries to maintain fluid balance and homeostasis. Serial measurements of both USG and PCV/TP can help the veterinary care team evaluate the effectiveness of fluid resuscitation efforts, as both levels should decrease as intravascular volume is restored and the interstitial fluid and ICF compartments are replenished.
Previous, Ongoing, and Anticipated Losses
Consideration of fluid losses is an important part of determining a fluid therapy plan. These losses may have occurred before presentation to the clinic—such as animals with a history of protracted vomiting or diarrhea—or may be anticipated after treatment has been instituted, as is often seen in cases of postobstructive diuresis in cats with urinary obstruction. These losses must be factored in when deciding the type, amount, and route of fluid therapy. When calculating fluid losses, veterinary technicians should include urination, defecation/diarrhea, vomiting, removal of effusions or gastric contents, fluid loss from drains, and insensible losses (such as from panting).
Ways to Provide Fluid Therapy
Even veterinary technicians who have been in practice for only a short while have likely seen fluids given several ways. Oral, subcutaneous, intravenous, intraosseous, and even intraperitoneal routes are all used, depending on the species receiving fluid therapy and why it is needed.
Oral Route
By far the simplest mode of fluid therapy, providing water per os can correct some conditions, including mild salt toxicity and mild cases of dehydration. Providing water via the oral route is as simple as offering the patient a bowl with a premeasured volume of water on a set schedule and measuring the amount consumed. However, in patients that have gastrointestinal pathology (i.e., parvovirus infection) or are unable to consume adequate amounts of water to maintain normal urine production or to establish and maintain fluid homeostasis, other means of fluid resuscitation must be used.
Subcutaneous Route
Subcutaneous fluids are a mainstay of veterinary therapy. Subcutaneous fluid administration is used for many disease conditions, including cases of mild vomiting and diarrhea or mild dehydration, or to support kidney function in animals with chronic kidney disease. It is relatively simple to provide fluids via the subcutaneous route, and many owners can be trained to provide this therapy at home, mitigating the need for hospitalization. As with other therapies given subcutaneously, it takes time for subcutaneous fluids to be absorbed into the bloodstream; thus the subcutaneous route is not appropriate to treat life-threatening conditions such as severe dehydration or shock.
Intravenous Route
IV fluid therapy is very common in veterinary practice and allows practitioners to restore intravascular volume, correct dehydration, and administer IV medications. IV catheter placement is a core nursing competency for veterinary technicians and allows for IV fluid therapy in emergency presentations and hospitalized patients alike. In addition, access to the vascular space allows for other therapies, including transfusions, medications, and parenteral nutrition.
In emergency situations or when a large volume of fluid is needed over a short amount of time, selecting a catheter with a large bore and a short length is preferable to allow for rapid infusion of fluids. This is a function of Poiseuille’s law, which governs the flow of fluid through a tube: essentially, the shorter the tube, the smoother the flow, and the larger the tube’s diameter, the faster the flow, meaning that large-bore, short catheters are the best choice when a large volume of fluid must be delivered quickly, such as in cases of hypovolemic shock.6,7 T-ports and additional tubing (e.g., extension sets) may decrease both the amount of fluid and the speed of delivery. In an emergency situation, it is best to minimize any extra IV accessories that might impede flow.
In addition to peripheral access, IV fluid therapy can be delivered through central line catheters. These catheters are longer than typical peripheral IV catheters and reach the central circulation via the vena cava. Central lines are commonly placed in the jugular vein, with the tip of the catheter sitting just outside the entrance to the right atrium to facilitate measurement of central venous pressures, if desired. Jugular central line catheters can be placed with a guidewire (i.e., Seldinger technique) or a peel-away introducer. They are available with multiple lumens to enable sampling, concurrent administration of incompatible fluids, and administration of hypertonic solutions that may cause phlebitis if given peripherally (e.g., dextrose concentrations >7.5%). The central circulation can also be reached with a long, through-the-needle catheter (e.g., Intracath) placed in either the lateral saphenous vein or the medial femoral vein or a peripherally inserted central catheter (PICC) in the same vessels. Because of their long length, smaller bore, and longer time usually required for placement, central catheters are not recommended for emergency fluid therapy, but can be maintained for long periods, making them well-suited to longer-term fluid therapy.
Intravenous Intraosseous Route
Intraosseus (IO) catheters are an excellent choice for providing drugs and fluids to patients in which IV access is difficult—if not impossible—to obtain in a timely fashion. Patients with severe hypotension or complete cardiovascular collapse (i.e., patients in cardiac arrest), that are severely dehydrated, or in which IV access is not obtainable (as in patients with edema, burns, thrombosis, or obesity) can benefit from placement of a catheter in the medullary cavity of a bone (IO). This route is also very useful in tiny patients, such as neonates and pocket pets (e.g., hamsters, gerbils). The materials are readily available in most, if not all, veterinary practices, and placement may mean the difference between life and death. The IO route is fast and has been proven8,9 to provide access to the central circulation comparable to the access provided by central venous catheterization, making it the first choice for administration of drugs and fluids when IV access cannot be achieved.
For all of the advantages of the IO route, there are several limitations. Fluid cannot be provided at a rate equivalent to that of IV access, and the needles are not designed for long-term use. Most sources1,2,4,7,10 recommend removal of IO access devices within 72 to 96 hours of placement to avoid the development of osteomyelitis or bone infections, as long as IV access can be obtained.
Monitoring
Veterinary technicians are responsible for providing therapies in as safe a manner as possible; this includes fluid therapy. Safety can be maintained with vigilant monitoring. To monitor a patient’s perfusion status, technicians should observe forward perfusion parameters (BOX 2). Normalization of these parameters is a good indication that fluid therapy is being provided successfully. In the laboratory, technicians can perform serial measurements of PCV/TP and USG. In patients that presented in a state of dehydration with increased PCV/TP, lowering of these values indicates a return to normal fluid levels in the intravascular space and an improvement in overall hydration. Increasingly dilute urine means that the patient’s kidneys have detected an increase in intravascular volume and a restoration of overall fluid balance.
One of the easiest and most sensitive ways to monitor fluid therapy in patients is with multiple weight checks throughout the course of therapy. Since TBW is 60% of a patient’s body weight, increases in any fluid compartment lead to a commensurate increase in the patient’s overall weight. However, an increase >10% from baseline admission weight should prompt an investigation of the possibility that the patient is becoming overhydrated, also known as becoming fluid overloaded.
Fluid overload is a major complication of fluid therapy and can lead to pulmonary edema, ascites, and peripheral edema with the potential for development of compartment syndrome. A patient who becomes tachypneic, develops clear nasal discharge, or is found to have crackles on thoracic auscultation while receiving fluid therapy should be suspected of becoming overhydrated. If these signs are noted, particularly in combination with an increase in body weight, IV fluid therapy should be stopped and the veterinarian should be notified immediately.11 Chemosis (swelling of the conjunctiva) is a late sign of fluid overload and requires urgent treatment (FIGURE 3), including cessation of IV fluids and potential administration of diuretic agents.

FIGURE 3. Swelling of the conjunctiva without signs of inflammation or irritation is known as chemosis. This is a late sign of fluid overload; it is incumbent on veterinary technicians to recognize earlier signs such as increased respiratory rate and effort, increased breath sounds (e.g., crackles), or clear nasal discharge.
Fluid Types Available
Several types of fluids are available, ranging from crystalloids to synthetic colloids to natural colloids (i.e., blood products). Each type has its place in the treatment of various conditions and pathologies found in veterinary patients. It is easiest to differentiate fluids based on their purpose: maintenance or replacement therapy. TABLE 1 outlines the components of common maintenance and replacement fluids available to veterinary practitioners in the United States. The resources listed in the Recommended Reading box can provide more detailed explanations of fluid types and their effects.
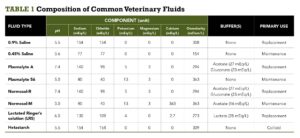
TABLE 1. Composition of Common Veterinary Fluids
Crystalloids
Patients presented as an emergency often require immediate intravascular expansion in the form of crystalloid boluses, or large volumes of crystalloid fluids. Crystalloid fluids move quickly from the intravascular space into other fluid compartments, primarily the intracellular compartment. Less than one-third of the crystalloid volume administered intravenously persists in the vasculature 1 hour after administration,4 making these fluids an excellent choice for treating dehydration and electrolyte derangements and correcting free water deficits.
Crystalloid fluids can be categorized as follows:
- Free water:5% dextrose in sterile water or 0.45% saline. This hypotonic (i.e., containing fewer solutes than ICF) solution replenishes the interstitial fluid and ICF compartments.
- Replacement solutions:These balanced, isotonic solutions are designed to replenish the ECF compartments, including increasing intravascular volume and restoring perfusion. Isotonic fluids contain a solute concentration that approximates that of ICF, and crystalloids that are considered “replacement” fluids (TABLE 1) have compositions that closely match the electrolyte balance and pH of ECF,1 making them ideal to replace losses from that fluid compartment (e.g., dehydration).
- Maintenance solutions:These balanced, isotonic solutions have less sodium and more potassium than replacement fluids and may be more suitable for long-term fluid therapy after restoration of intravascular volume and correction of electrolyte derangements. Maintenance fluids are rarely used alone—they are usually combined with a ratio of 0.9% sodium chloride1 (aka “normal” or “isotonic” saline) to more closely match the composition of the fluid in the intravascular space, preventing unwanted fluid shifts between compartments.
- Hypertonic solutions:7% to 23.4% saline. These fluids contain a solute concentration higher than that of ICF and rapidly expand intravascular volume by drawing water from the interstitial and intracellular compartments. Because of this oncotic pull, hypertonic solutions should never be used in cases of severe dehydration.
Colloids
Many practitioners also use colloids (either synthetic or natural) in an emergency to expand the intravascular compartment without the risk of fluid overload posed by infusing large volumes of crystalloid fluids. Colloids contain large, osmotically active particles that work to hold fluid in the vasculature after administration.
Synthetic colloids are fluids with large molecules designed to provide oncotic pressure support within the intravascular space. Natural colloids are blood products such as whole blood, packed red blood cells (pRBCs), plasma, and albumin. Whole blood and pRBCs have the added benefit of providing oxygen-carrying capacity, helping to prevent and treat hypoxia.
The use of colloids is highly controversial in human medicine and becoming so in veterinary medicine as well,12 with recent research13 implicating a link between the use of a synthetic colloid and the development of acute kidney injury in dogs.
Developing and Implementing a Fluid Therapy Plan
There is a helpful guideline when it comes to fluid therapy: Replace like with like. This means if a patient has lost blood, that fluid should be replaced with plasma, pRBCs, or whole blood. If a patient has lost body fluids through diarrhea, vomiting, or excessive urination, replacement should be with similarly constituted isotonic crystalloid fluids. While development of the fluid plan is ultimately the veterinarian’s purview, it is important for veterinary nurses and technicians to understand the fluids available and for what conditions they might be used in clinical practice.
Fluid therapy in the veterinary hospital or clinic has 3 primary phases, which can overlap and alternate, depending on how a patient presents and the progression of its disease process. The resuscitation phase refers to correcting shock and other life-threatening fluid deficits; the replacement phase is the time taken to replace dehydration deficits; and the maintenance phase covers fluids provided during hospitalization to support and maintain homeostasis. BOX 3 provides examples of fluid choices in some specific disease processes.
BOX 3 Appropriate Fluid Choices for Selected Disease Processes
- Cardiac disease:Low-dose maintenance crystalloid, such as 0.45% saline with dextrose (may require potassium and or magnesium supplementation)
- Vomiting/diarrhea:Replacement crystalloid, such as lactated Ringer’s solution, Normosol-R, or
Plasmalyte-A - Diabetic ketoacidosis:Replacement crystalloid, such as lactated Ringer’s solution, Normosol-R,
Plasmalyte-A - Hemorrhage: Natural colloid, such as plasma, whole blood, pRBCs
The amount of fluid to be provided to a patient must be calculated carefully, taking into account the need for intravascular volume expansion, the profundity of perfusion deficits, the degree of dehydration, and the severity of electrolyte derangements, among other considerations. BOX 4 lists common fluid therapy calculation formulas.
BOX 4 Fluid Therapy Formulas
Calculation of Dehydration Deficit1
Body weight (kg) × % dehydration as a decimal = liters of fluid required to correct dehydration
Calculation of Maintenance Fluid Requirements*
Dogs: Body weight (kg)0.75 × 132 = 24-hour fluid requirement in milliliters
Cats: Body weight (kg)0.75 × 80 = 24-hour fluid requirement in milliliters
Ongoing losses (e.g., from diarrhea, vomiting, or polyuria) must be calculated and added to the total maintenance requirement obtained from these formulas.
Fluid Therapy Procedure in Dogs
Fluid therapy is individualized and tailored to each condition, as well as the canine. The location in which the fluid is infused, fluid composition, rate, and fluid volume are dictated by the needs of the patient. Fluid therapy can be administered to a dog intravenously (through the vein), subcutaneous (under the skin), intraosseous (through the bone), or intraperitoneal (through the abdomen). In general, the procedure for administration of fluid therapy is ideally the same with the placement of a catheter and rate of administration being the only differences.
- The veterinarian will determine the site of administration (intravenously, subcutaneous, intraosseous, or intraperitoneal).
- The type of fluid will be determined based on the dog’s condition (Isotonic Crystalloids, Colloids, Hypertonic Saline) and an IV bag will be prepared.
- The fluid rate will be calculated.
- The size of the catheter will be determined, placing the largest catheter possible to provide adequate rates of fluid.
- The fluid therapy catheter will be prepped for placement. Saline solution is run through the port (the connective device for the catheter) and it is swabbed with alcohol.
- The hair will be clipped and cleansed to perform a sterile preparation.
- The catheter used for fluid therapy is equipped with a needle to allow penetration of the skin. The catheter will pierce the skin, the plastic catheter will be pushed into the skin (or vein) as the needle is pulled out.
- The end of the catheter is capped off to prevent bleeding as it is taped into place.
- Once the catheter is taped into place, the cap is removed and the IV line is connected.
- An IV bag full of the pre-prepared fluids will be connected to the line and the valve will be opened to the pre-calculated flow rate.
Parenteral fluid therapy
Parenteral fluid therapy is the most common therapeutic intervention performed in veterinary emergency practice. A thorough understanding of the indications for the use of parenteral fluids, the types of therapeutic fluid available, and the most appropriate protocol for their administration is mandatory both to maximize the benefit and minimize the potential harm associated with this therapy.
Hypovolaemia and dehydration are the most common indications for the use of fluid therapy and it is essential to understand their differences with respect to pathophysiology and clinical assessment in order to administer appropriate fluid therapy (see Ch. 2). This chapter focuses on the different types of parenteral fluid commonly available in nonreferral emergency practice and their appropriate use in hypovolaemia and dehydration.
Types of Parenteral Fluid
Crystalloids
Crystalloids Crystalloids are solutions that are isotonic with plasma and contain sodium as the major osmotically active particle. Lactated Ringer’s Solution, 0.9% Sodium Chloride (Normal Saline), and Normosol-R are isotonic crystalloid solutions. The most common uses of these solutions are for maintenance of normal hydration, replacement of hydration deficits (including use during anesthesia,) and with shock therapy. Crystalloids are also useful for the infusion of medications such as potassium chloride (KCl). It is important to remember that when crystalloids contain additives, they should never be used for fluid boluses. KCl in particular can result in arrhythmias and even death when administered rapidly. This is due to the fact that rapid infusion of potassium can induce cardiac arrest. The most common routes of crystalloids are subcutaneous (SQ), intravenous (IV), and intraosseous (IO).
Crystalloids are electrolyte solutions that can pass freely out of the bloodstream through the capillary membrane. Crystalloid solutions are described as isotonic, hypertonic or hypotonic based on how their tonicity compares to that of plasma. The tonicity is related to the sodium concentration and it is the tonicity that determines how the crystalloid solution is distributed between fluid compartments following administration into the bloodstream.
The two most commonly used crystalloid solutions are buffered lactated Ringer’s solution (Hartmann’s solution, compound sodium lactate) and 0.9% sodium chloride (normal strength or physiological saline). Both these solutions are examples of replacement isotonic crystalloids as their tonicity and electrolyte composition are similar to that of extracellular fluid. Following intravascular administration, these fluids equilibrate relatively quickly with the interstitial space and 75–85% of the administered volume is likely to have left the bloodstream 30–60 min after infusion. This is why large volumes are required to expand the intravascular compartment effectively in hypovolaemia and is also the reason why these solutions are used to replenish extravascular fluid losses in dehydration (see below).
Hypertonic (e.g. 7.2–7.5% sodium chloride (hypertonic saline)) and hypotonic (e.g. 0.45% sodium chloride (half strength saline)) crystalloid solutions are also available but their use is much less commonly indicated. Hypertonic saline administration causes plasma volume expansion mainly by drawing water out of cells into the extracellular space down an osmotic gradient. Most of this fluid remains in the interstitial space but a proportion diffuses into the vasculature. The recommended dose is 4 ml/kg i.v. (dogs 4–7 ml/kg, cats 2–4 ml/kg) over a minimum of 5 minutes and a rapid though short-lived effect is typically seen (within 5 minutes). Hypertonic saline is indicated in volume resuscitation, especially in large or giant breed dogs where rapid administration of large volumes of isotonic crystalloids may be impossible. Administration of hypertonic saline must be followed by the use of a replacement isotonic crystalloid due to the osmotic diuresis and rapid sodium redistribution that occur with this treatment. Hypertonic saline is often administered in combination with a colloid solution to prolong intravascular volume expansion. Hypertonic saline is also indicated in the treatment of raised intracranial pressure, especially with concurrent hypovolaemia, where it causes fluid to move out of the brain parenchyma and into the vasculature (see Ch. 28).
Hypotonic saline is most often used in combination with 0.9% sodium chloride to correct hyponatraemia gradually. It is also occasionally used in dehydrated animals with cardiac disease to provide rehydration while limiting the amount of sodium administered.
Maintenance Rates: There are several formulas for calculating maintenance fluid rates in dogs and cats. The best or most accurate calculation for determining the crystalloid maintenance rate for dogs is the following body surface formula: (kg x kg x kg) √√ x 132 ÷ 24 = ml/hr
Example: A 40-kg dog is presented to your hospital for treatment of dehydration caused by vomiting; the maintenance rate is calculated as:
(40 x 40 x 40) √√ x 132 ÷ 24 = 87 ml/hr
The calculation for crystalloid maintenance rate for cats is the following formula:
(kg x kg x kg) √√ x 70 ÷ 24 = ml/hr Another standard formula often used is 60mL/kg/day for maintenance fluids. For the 40-kg dog: 60 mL X 40 kg = 2400 /24 hours = 100 mL/hr
While there are variations in fluid rate calculations, the fluid rate should always be determined based on the hydration status and medical condition of the patient (i.e. ongoing losses, deficits, and maintenance requirements).
Shock Doses: During treatment for shock, LRS and Normosol-R are the preferred isotonic crystalloids, administered at a rate of 90 ml/kg in dogs and 45 ml/kg in cats. Often the shock dose is given in ¼ boluses (a quarter shock dose initially) and the patient is then reassessed. Once the patient has stabilized, a new rate is calculated in order to continue to correct for fluid deficits. Typically, the goal is to stabilize and rehydrate the patient over a 24-hour period.
Clinical Tip
- A 0.18% sodium chloride (with 4% glucose) solution is commercially available. This solution is markedly hypotonic compared to plasma and in the author’s opinion there is no indication for its routine use. It may occasionally be required to treat animals with severe acute hypernatraemia but this is rare in companion animals. If this solution is used, animals must be monitored very closely for electrolyte abnormalities (hyponatraemia in particular) that may be severe with potentially very serious clinical consequences.
- The author is aware of cases in which this solution has been used as a convenient way of administering intravenous glucose supplementation. However, other proprietary glucose solutions (e.g. 5% glucose in 0.9% sodium chloride solution) are available and glucose solutions can also readily be formulated (see Appendix 1, Drug Formulary). The author would therefore strongly discourage the use of the 0.18% sodium chloride solution as a means of administering glucose supplementation.
Dextrose Solutions
Dextrose solutions are formed when dextrose is added to a crystalloid. Dextrose can be used to provide free water to replace insensible losses or for correction of hypernatremia resulting from a water deficit. When added to a crystalloid, dextrose can be used to provide an intracellular carbohydrate source in septic patients and aids in correction of hypoglycemia. These solutions should not be used as maintenance fluids because their administration will lead to the dilution of electrolytes.
Administration routes should be exclusive to IV and IO. Administering dextrose solutions into subcutaneous tissues causes electrolytes to move into these tissues, leading to a decrease in circulating blood volume and resulting in tissue necrosis. It is also important to remember that these solutions are ideal for bacterial growth; therefore, aseptic technique must be used. Rates of administration will vary depending on the concentration and purpose.
Synthetic Colloids
Synthetic colloids act primarily to expand plasma volume. They are useful as either resuscitative or replacement fluids and can be given as a bolus if the patient has poor perfusion due to hypovolemia. Hetastarch, Dextrans 40, and Dextrans 70 are the most commonly used synthetic colloids. Routes of administration are limited to IV and IO. The synthetic colloid maintenance rate for Hetastarch is 20 ml/kg/day. For the treatment of hypotension, 5-10 ml/kg is given as a bolus. Dextrans 40 and Dextrans 70 are given at a rate of 2 ml/kg/hr.
Colloids
Colloids are used for the relative expansion of the interstitial space in the event of a plasma volume deficiency resulting from traumatic or septic shock and for replacement of lost blood volume. Colloid solutions include human and canine albumin, fresh frozen plasma, and whole blood. Colloids are most commonly given IV but can be administered IO if venous access cannot be achieved. The rate of administration depends on the clinical status of the patient and the reason for the transfusion.
Signs of Dehydration
5% – 6% dry or “sticky” oral mucous membranes 6% – 8% mild to moderate decrease in skin turgor, dry or “sticky” oral mucous membranes, sunken eyes 10% – 12% marked decrease in skin turgor, dry mucous membranes, sunken eyes, weak and rapid pulses, slow capillary refill time, moderate to marked mental depression.
Signs of Overhydration
- Serous nasal discharge • Subcutaneous edema • Increased urine output • Ascites • Coughing / pulmonary edema • Increased respiratory rate
Uses of isotonic crystalloid solutions
Isotonic crystalloid solutions are used in two main ways:
- High rate isotonic crystalloid therapy is used to treat hypovolaemia either alone or in combination with other fluid types (colloid, hypertonic saline)
- Low rate isotonic crystalloid therapy is used to treat dehydration.
Synthetic colloids
Colloid solutions consist of large (macro) molecules that do not readily leave the intravascular space (through capillary pores) and are hyperoncotic relative to normal animals. Synthetic colloids therefore draw fluid into and hold fluid in the vasculature, causing plasma volume expansion. Commercially available synthetic colloid preparations are often made up in a 0.9% sodium chloride solution. Nonsynthetic (natural) colloid solutions that are currently used therapeutically include plasma and human serum albumin solutions.
The three types of synthetic colloid solution currently in veterinary use are:
- Hydroxyethyl starches: tetrastarch (e.g. Voluven 130/0.4®, Fresenius Kabi); hetastarch (e.g. Hetastarch 600/0.7®, Baxter); pentastarch (e.g. HAES-Steril 200/0.5®, Fresenius Kabi)
- Gelatins (e.g. Gelofusine®, Braun; Haemaccel®, Intervet)
- Dextrans (e.g. Dextran-70®, Pharmacosmos).
The volume and duration of plasma expansion that follows colloid administration depend in part on the specific colloid used (its colloid osmotic pressure (COP)), as well as the dose given and the species in question.
Indications for synthetic colloid use
Synthetic colloids are usually used in hypovolaemic patients in one of two scenarios:
1 When fluid resuscitation with an isotonic crystalloid solution has proved to be ineffective; this is because colloids are more efficient at intravascular volume expansion.
2 When there are specific reasons that warrant the inclusion of a colloid; this might be the case for example in animals that are hypoproteinaemic (e.g. from gastrointestinal or renal loss). Isotonic crystalloids will remain in the circulation for even less time in these animals as plasma proteins (albumin in particular) are partly responsible for retaining these fluids in the intravascular space.
Synthetic colloids are not generally used alone when employed in the treatment of hypovolaemia and are typically discontinued earlier on than the replacement crystalloid solution. It is much less common for patients to remain on long-term colloid therapy (although on-going hypoproteinaemia or the presence of systemic vasculitis or capillary-leak syndrome for example may warrant this treatment).
Adverse effects of synthetic colloids
The most important adverse effect to consider when using synthetic colloids is their effect on coagulation that is likely to be multifactorial in origin. Allergic reactions are also rarely identified.
Haemoglobin-based oxygen-carrying solutions
Haemoglobin-based oxygen-carrying solutions (HBOC) are not blood replacement solutions. They increase plasma haemoglobin concentration and therefore oxygen-carrying capacity but do not contain other blood constituents. The only HBOC currently available for veterinary clinical use is Oxyglobin® (Biopure Corporation; www.biopure.com). This solution is based on polymerized modified bovine haemoglobin and is administered using standard intravenous fluid administration sets. It is currently only licensed for use in dogs but has been used extensively off-licence in cats with great success.
The main indication for Oxyglobin® is in euvolaemic anaemia where it can act as a substitute for the deficient red blood cells and allow improved tissue oxygenation. Unlike with blood transfusions, there are no cellular antigens in Oxyglobin® so typing and crossmatching do not need to be performed. The product as supplied in foil by the manufacturer also has a long shelf-life of 3 years. Oxyglobin® is used in these cases to support the patient while diagnosis is achieved and treatment is instituted and given time to take effect.
Clinical Tip
Administration of Oxyglobin® typically causes a fall in packed cell volume (PCV) due to intravascular volume expansion. Ideally, therefore, plasma haemoglobin concentration should be used to monitor increase in oxygen-carrying capacity in lieu of packed cell volume. An approximately equivalent PCV can be calculated as follows:
However, a haemoglobinometer is required to measure free plasma haemoglobin concentration as in-house machines typically calculate haemoglobin concentration from haematocrit. Regardless of whether a haemoglobinometer is available or not, positive clinical response to treatment is the best guide of effective therapy.
As Oxyglobin® contains large molecules it is also a potent colloid solution and can therefore be used very effectively to provide intravascular volume expansion in animals with hypovolaemia. Despite the unique oxygen-carrying benefits of this modified biological colloid, the much greater cost of Oxyglobin® over other available colloids means that its use in hypovolaemia is typically restricted to animals that have suffered significant blood loss.
Clinical Tip
- The volume expanding properties of Oxyglobin®must be borne in mind when it is used to treat anaemia in patients without hypovolaemia as there is a very real risk of fluid overload if inappropriately high rates are used, especially in cats.
- Typical rates for Oxyglobin®use in euvolaemic anaemic patients are 0.5–1 ml/kg/hr in cats and 1–2 ml/kg/hr in dogs.
- Caution is also warranted in disease states associated with an increased risk of fluid overload such as cardiac disease, pulmonary parenchymal disease and cerebral oedema
As with all colloids, Oxyglobin® will interfere with serum total solids measurement via refractometry and results must be interpreted cautiously. Oxyglobin® will also interfere with colorimetric serum biochemistry analysis although the parameters affected will depend on both the analyser and the methodology. Peripheral blood smear evaluation is not affected.
The Fluid Plan
On the basis of the physical examination, and subsequently other findings, it should be possible to answer the following questions (see Ch. 2):
- Is the animal hypovolaemic and if so, is this mild, moderate or severe?
- Is the animal dehydrated and if so, what is the estimated percentage dehydration?
Hypovolaemia
The basic objective is to restore the effective circulating intravascular volume and thereby restore adequate tissue perfusion. Appropriate fluid therapy is therefore provided until end-points suggestive of acceptable systemic perfusion are achieved. This volume expansion is performed over a short period of time – usually a few minutes to an hour but sometimes longer – and may involve the use of both isotonic crystalloids and colloids including Oxyglobin® (plus whole blood and hypertonic saline if available). Isotonic crystalloids are the first choice in the majority of cases.
How?
Hypovolaemia is treated via the intravenous route using one or more of the shortest but largest bore catheters possible. If a peripheral vein cannot be catheterized and a central venous catheter is not available or inappropriate, the intraosseous route may be used initially.
How much and for how long?
Isotonic crystalloids
See Table 4.1 for guidelines for initial rates of isotonic crystalloid fluid therapy in dogs and cats with uncomplicated hypovolaemia. Initial boluses are usually given over 15–20 minutes. For some bigger dogs the use of a pressure infusor (Figure 4.1) around the crystalloid bag can be invaluable in delivering the fluid within a suitable period of time.
Table 4.1 Guidelines for isotonic crystalloid therapy for hypovolaemia in dogs and cats. Initial boluses are usually given over 15–20 minutes
Pros of Colloid Administration
The use of colloids to expand intravascular volume, maintain intravascular volume and reduce third spacing may be beneficial in the setting of protein loss, endothelial leakage, or decreased protein production. Colloids may assist in increasing intravascular volume via their contribution to oncotic pressure. A study published by Silverstein et al. (2005) described the efficiency ratio (i.e. the ratio of the increase in blood volume to the amount infused) of various crystalloids and colloids administered to beagles in a randomized crossover design. Both HES and dextrans had an efficiency ratio of approximately 1.0 immediately post-infusion and this ratio increased to approximately 1.4 at 30 minutes post-infusion. By comparison, 0.9% saline had an immediate efficiency ratio of 0.8 that decreased to 0.4 at 30 minutes post-infusion, thus demonstrating the persistent increase in blood volume after synthetic colloid use.
Cons of Colloid Administration
A major concern with starch solutions is their effect on coagulation, acute kidney injury, fluid overload and anaphylaxis. HES has been shown to decrease levels of von Willebrand’s factor and factor VIII beyond those expected by dilution alone and bind to the surface of platelets blocking receptor sites to interfere with fibrin clot stabilization. This effect appears to be most pronounced in HES preparations of higher molecular weights with greater degrees of substitution. This is reflected in the maximal daily dosages listed in Table 1, in which tetrastarch (lowest molecular weight) has the highest recommended limit.
NB- Isotonic Fluids, Hypotonic Fluids, and Hypertonic Fluids
There is a wide variety of fluids are available for use by the veterinary practitioner. A crystalloid fluid contains crystals or salts that are dissolved in solution. Specific crystalloid fluids are indicated in certain disease states, and may be contraindicated in others. Therefore, whenever a crystalloid fluid is used, one must carefully consider it to be another drug in the armamentarium, and justify its use or potential disuse in each patient. Basic categories of crystalloid fluids include isotonic, hypotonic, and hypertonic solutions, depending on the concentration and type of solute present relative to normal body plasma. Isotonic fluids have tonicity, or solute relative to water, similar to that of plasma. Examples of isotonic fluids include 0.9% (normal) saline, Lactated Ringer’s solution, Normosol-R, and Plasmalyte-A. Isotonic fluids are indicated to restore fluid deficits, correct electrolyte abnormalities, and provide maintenance fluid requirements. Hypotonic solutions are fluids whose tonicity is less than that of serum. Examples of hypotonic fluid solutions include 0.45% saline, 0.45%NaCl + 2.5% dextrose, and 5% dextrose in water (D5W). Hypotonic fluids are indicated when treating a patient with diseases processes that cause sodium and water retention, namely, congestive heart failure and hepatic disease. Infusion of hypotonic fluids is also indicated when severe hypernatremia exists, and you need to slowly correct a free water deficit. To calculate a patient’s free water deficit, use the following formula: Free water deficit = 0.4 x lean body weight x [patient serum Na/140 – 1] The free water deficit should be corrected slowly, to not cause iatrogenic cerebral edema. Ideally, the patient’s sodium should not decrease by more than 15 mEq/L during a 24-hour period. Hypertonic solutions act to draw fluid from the interstitial fluid compartment into the intravascular space to correct hypovolemia. Their use is absolutely contraindicated if interstitial dehydration is present. Hypertonic solutions such as 3% or 7% saline have solute in excess of fluid relative to plasma. Hypertonic saline should be administered in bolus increments of 3 – 7 ml/kg as a rapid infusion. Because the net effect of hypertonic saline solution lasts only approximately 20 minutes, hypertonic saline must always be infused along with a crystalloid solution to prevent further interstitial dehydration.
IV Fluid Therapy Calculations
The basics:
• Maintenance fluid rate for an adult dog or cat is estimated as 2mL/kg/hr OR 50mL/kg/24 hours • e.g. 35kg dog: Maintenance = 35kg x 2 = 70mL/hour or 50mL x 35kg = 1750mL/24 hours • Maintenance fluid rate for puppy or kitten may be estimated as 3-4mL/kg/hr •
Fluid deficit: • If present, the fluid deficit needs to be calculated and this can be done by estimating the percentage dehydration: • e.g. A 35kg dog that is estimated to be 8% dehydrated • Percentage dehydration is estimated to be 8% of the body weight and then this is converted into fluid units: • e.g. 35kg x 0.08 = 2.8kg • 1kg = 1L, therefore the dog has a 2800mL fluid deficit • If the fluid lost is to be replaced over 24 hours, the maintenance requirement is added to the fluid deficit to work out the total amount to be given over a 24 hour period: • e.g. 1750mL + 2800mL = 4550mL over 24 hours • In a severely dehydrated animal the deficit may be replaced over 24 hours, but at times it may be appropriate to ‘front-load’ e.g. 30% in the first 3 hours, though more of less may be appropriate depending on the case and response to treatment. • If a patient is in shock, generally a bolus of fluids will be given over a short period of time rather than increasing the fluid rate so that the fluid deficit is replaced more quickly. Ongoing losses: • Fluid rates must be adjusted according to the ongoing losses such as vomiting, diarrhoea, haemorrhage. • The volume of fluid that is being lost should be estimated in each case and then adjusted depending on disease progression and clinical assessment. • Once the overall fluid rate per hour has been calculated, taking into consideration maintenance, deficit and on-going loss, this information can be entered into an infusion pump if this is available. If not a drip rate needs to be calculated in mL/minute: • e.g. A dog needs 116mL/hour • 116mL/hour/60 = 1.93mL/minute • The amount needed per minute then has to be multiplied by the drops/mL that the giving set delivers. This can be found on the giving set. Use this to convert the amount needed in mL per minute to the number of drops per minute. • e.g. A 20drops/mL giving set • 1.93mL/min x 20 = 38 drops per minute • Set up the giving set to deliver 38 drops per minute – To check, the drip rate can be measured over a shorter period of time – e.g. 19 drops over 30 seconds, ~10 drops over 15 seconds • Note: Fluid administration rates can be controlled more accurately using an infusion pump. • Current practice for fluid administration during anaesthesia: • 2-4mL/kg/hr (healthy animal, minimal fluid losses e.g. blood loss or evaporation) • Faster rates may be required for individual animals i.e. for different cases and situations.
EXAMPLES-
1.Calculate the drip rate required for a 6 year old female DSH undergoing a routine dental. She weighs 3.4kg and otherwise healthy. The giving set delivers 60 drops/mL.
- 3.4kg x 4mL/hour = 13.6 mL/hr • 13.6mL/hour / 60 = 0.226mL/minute • 0.226mL/minute x 60drops/mL = 13.5 drops/minute = 0.225 drops/second = approx. 1 drop every 5 seconds
- A 15 week old, 6.8kg puppy has been admitted with vomiting and diarrhoea that have been present for 2 days. The puppy is 6% dehydrated which needs to be corrected over 24 hours. What fluids will be used initially for the puppy and what drip rate would be used? The giving set delivers 20 drops/mL.
- Maintenance = 3mL/kg/hr x 24 hrs = 72mL/kg/24hrs = 489.6mL • Deficit = 6.8kg x 0.06 (% dehydration) = 408mL • Total over 24 hours = 489.6mL + 408mL = 897.6mL • 897.6mL /24 hrs = 37.4mL/hr • 37.4mL /60min = 0.623mL/min • 0.623mL/min x 20drops/mL = 12.5 drops/min • 0r if you had used 4mL/kg/hr. Answer: 15 drops/min = 0.25 drops/second = approx. 1 drop every 4 seconds If the puppy continues to vomit and have diarrhoea, these ongoing losses with need to be factored in to the fluid therapy plan. • N.B. It is important to monitor the response to treatment and alter the fluid rate accordingly; the initial calculations are only the starting point.
- A dog is admitted following an road traffic accident (RTA) and is in hypovolaemic shock. The dog weighs 35kg and you decide that it needs a bolus of fluids. You decide to give it a 30mL/kg bolus over 20 minutes. The giving set delivers 20 drops/mL. What would the drip rate be? What could be done to try to ensure the animal received this volume in the time required?
- 35Kg x 10mL/kg = 350mL over 10 minutes • 350mL x 6 = 2100mL over 1 hour • 2100 /60 = 535mL/minute • 35mL/minute x 20 drops/mL = 700 drops/minute = 11.6 drops every second • It would be difficult to accurately set a giving set to this speed and most infusion pumps would not work at this speed – so if this is what is required the bag would need to be squeezed. Another options would be to place another i.v. catheter and run 2 bags of fluid in at once.
- A 4.7kg, 9 year old MN DSH cat has been admitted with a severely distended and painful bladder due to a urethral obstruction. You successfully catheterise the urethra but the cat is 10% dehydrated. He has a high (raised) potassium and is depressed. What would be the setting of an infusion pump to deliver a bolus of 25mL/kg over 30 minutes? What fluid type would be most appropriate in this case? •
Either 0.9% NaCl or Hartmans would be suitable in this case. • 4.7kg x 25mL/kg = 117.5mL over 30 minutes • 117.5mL x 2 = 235mL/hr • Enter 117.5mL under VTBI (volume to be infused) on the infusion pump N.B. Normally the value would be rounded up or down i.e. 117.5 set as 120mL
https://www.pashudhanpraharee.com/concept-of-fluid-therapy-in-animals/
REFERENCE-ON REQUEST