Leptospirosis: A Nefarious Zoonotic Disease in Humans and Livestock
|
1Indian Veterinary Research Institute, Bengaluru-Karnataka
2 Department of Epidemiology & Public Health, Central University of Tamil Nadu
3Project Directorate of Foot and Mouth Disease, Bengaluru-Karnataka
4Indian Veterinary Research Institute, Palampur-Himachal Pradesh
Synonyms: Akiyami (autumn fever), Cane-cutter’s disease, Nanukayami (seven day fever), Schlammfieber (mud fever), Swine-herd’s disease
Intoduction
Leptospirosis is a spirochaetal infection that possesses a broad host range affecting almost all mammals. It is a zoonotic disease of ubiquitous distribution, with a higher incidence in tropical than in temperate regions. In the developed world, most cases that occur are associated with recreational activities such as canoeing, rafting or swimming in contaminated water. Most countries in the South East Asia region are endemic to leptospirosis. In developing countries, sugar cane workers and rice field workers are at maximum risk of exposure since they walk barefooted on puddle of water contaminated with urine of reservoir hosts. Black rat (Rattus rattus) and Brown rat (Rattus norvegicus) are the reservoir hosts for leptospirosis and these rodents excrete leptospires through urine and contaminate the environment.
Morphology
Leptospires are flexible helical rods that are actively motile. They are too thin to be seen under light microscope and are best visualized under dark ground microscope. They cannot be stained readily with aniline dyes and can be stained only faintly by Giemsa stain. Leptospires are best stained by silver impregnation techniques such as Fontana Staining and Levaditi Staining. The size of the cell ranges from 6-20 μm in length and about 0.1 μm in thickness. Freshly isolated pathogenic leptospires tend to be shorter and tightly coiled than laboratory strains that have undergone repeated subcultures.
Culture Media:
Rabbit serum based culture media such as Korthof’s medium, Fletcher’s medium and Stuart’s medium are used for the isolation of leptospires from the clinical specimens and for the maintenance of leptospires but not for the preparation of antigens for MAT. Ellinghausen-McCullough-Johnson-Harris (EMJH) medium is the most popular medium widely used for isolation, maintenance, preparation of antigens for MAT as well as for growing leptospires in bulk.
Epidemiology
Transmission of leptospirosis can be direct or indirect. Direct transmission occurs when leptospires from tissues, body fluids or urine of acutely infected or asymptomatic carrier animals enter the body of the new host and initiate infection. Direct transmission among animals can be transplacental, by sexual contact or by suckling milk from infected mother. Direct transmission from animals to human beings is common in occupations that involve handling of animals or animal tissue such as butchers, veterinarians, cattle and pig farmers, rodent control workers etc.
Indirect transmission occurs when an animal or human being acquires leptospirosis from environmental leptospires, originating in the urine of excretor animals. It is considered that the most common portal of entry of leptospires into the host body is through intact skin. Leptospirosis is a known health hazard of rice farmers in countries such as Indonesia and Thailand. Ingestion of sewage contaminated drinking water has occasionally been reported to cause leptospirosis. Leptospirosis has been recognized as a potential hazard of water sports and other recreational activities that expose people to contaminated waters. Swallowing water while swimming was significantly associated with leptospirosis.
Human-to human transmission through breast-feeding has been recorded. However this mode of transmission is not of much epidemiological importance. Leptospirosis has now been recognized as a possible sequel of natural disasters such as cyclones and floods as people and animals are exposed to wet environments for prolonged periods of time
Leptospirosis in Human beings:
In humans, Weil’s disease refers to a syndrome of icteric leptospirosis with renal involvement. Another clinical form is severe pulmonary haemorrhage. Other complications include myocarditis, meningitis, acute respiratory failure, and renal failure. Uveitis is a late complication of leptospirosis. Pulmonary haemorrhage is the most fatal complication in leptospirosis. In Andaman, a high case fatality ratio has been observed amongst patients who develop pulmonary haemorrhage as compared to patients with other clinical presentations. The other most common fatal complication is renal failure. It was observed that a significant proportion of cases with renal failure attending Nephrology Department in Chennai, India had leptospiral etiology. Myocarditis may some times cause intractable hypotension and cardiac arrhythmias and might become fatal. Other complications such as meningitis rarely become fatal.
Leptospirosis in Dogs:
Leptospirosis is contracted by dogs when their mucous membranes or skin with wounds/ cuts come in contact with infected urine, urine contaminated fomites, contact with rodents, bite from an infected animal, eating infected carcasses, and rarely through breeding. Vertical transmission occurs when Leptospires gets passed through placenta from bitch to puppies. Serovars Canicola and Icterohaemorrhagiae are commonly isolated from dogs. Infected dogs may suffer illness referred as Stuttgart Disease characterized by dehydration, loss of appetite, lethargy, bleeding disorders, such as blood-tinged vomit, urine, faeces (stool) or saliva, nose bleeds and pinpoint red spots (visible on gums and other mucous membranes), jaundice (yellowing of the skin and mucous membranes) and painful inflammation within the eyes. Surviving dogs develop kidney failure due to chronic nephritis with or without liver failure and shed leptospires in their urine for prolonged period of time.
Leptospirosis in Pigs:
Pig is a reservoir host for Leptospira pomona, L. tarassovi, L. bratislava and L. muenchen. It is not a reservoir host for L. icterohaemorrhagiae, L. canicola and L. hardjo but can be infected with these serovars from urine of rodents, dogs and cattle respectively. Pigs are incidental hosts i.e. does not perpetuate the infection and is only responsible for minimal spread. L. pomona causes reproductive problems in sows. L. pomona cause abortion, stillbirths or the birth of live, weak piglets, kidney lesions (white spots) in grower/finisher pigs and rarely fever, jaundice and death in young piglets. L. tarassovi causes similar syndrome to L. pomona but tends to be milder. L. bratislava/muenchen permanently inhabit the fallopian tube of sows and the reproductive organs of boars and they are spread in semen.
Leptospirosis in Horses:
The most common causes of equine leptospirosis are serovars pomona and grippotyphosa. Antibodies to serovar Bratislava are also reported and horses are maintenance host for this organism. Clinical leptospirosis in horses is associated with abortions, systemic illness in foals, and recurrent uveitis. Leptospirosis is responsible for 2–4% of all equine abortions annually. In foals, acute leptospirosis is typical with hemolysis and vasculitis with petechial hemorrhages on mucosal surfaces, hemoglobinuria, anemia, icterus, conjunctival suffusion, depression, and weakness. Renal failure and hepatopathy may also occur. Typically, uveitis develops 2–8 months following the initial infection. Leptospires have been identified in aqueous humor of >50% of horses with recurrent uveitis.
Leptospirosis in Cattle
Leptospiral serovars of major economic importance in cattle are Hardjo and Pomona with serovars Grippotyphosa, Bratislava, Icterohaemorrhagiae and Canicola occasionally implicated. The most common cause of leptospirosis among cattle throughout the world is serovar hardjo, for which cattle are the maintenance host. Severe acute disease occurs in calves infected with incidental serovars, particularly serovar pomona. Clinical signs include high fever, hemolytic anemia, hemoglobinuria, jaundice, pulmonary congestion, occasionally meningitis, and death. In lactating cows, incidental infections cause agalactia with small quantities of blood-tinged milk. A less severe form of this “milk drop syndrome” may occur in hardjo-infected lactating cows in the absence of other clinical evidence of infection. The chronic phase of disease is associated with fetal infection in pregnant cows presenting as abortion, stillbirth, or birth of premature and weak infected calves. Abortions associated with incidental host infection tend to occur late-term and in groups or so-called “abortion storms.” In contrast, abortions occurring after infection with serovar hardjo tend to be more sporadic and can occur mid- to late pregnancy.
Leptospirosis in Sheep and goats
Leptospirosis in sheep and goats is similar to the disease in cattle. It is characterized by fever and anorexia and, in some animals, jaundice, hemoglobinuria or anemia. Abortions, stillbirths, weak lambs or kids and infertility can also be seen, either with or without other clinical signs. Clinical disease is relatively uncommon in sheep.
Diagnosis
Demonstration of the organism in body fluids
The demonstration of leptospires in blood and milk of animals showing clinical signs suggestive of acute leptospirosis is considered to be diagnostic. However, isolation from blood is not often successful because bacteraemia is transient and not always accompanied by clinical signs. Dogs are often treated with antibiotics before samples are collected for testing for Leptospira, which further decreases the likelihood of identifying the agent in blood. The isolation of Leptospira organisms is the direct proof for the detection of leptospira in body fluids such as urine and blood. Isolation of the organisms is achieved in liquid EMJH media in the first 10 days in blood samples during the leptospiraemic phase. After 10 days, isolation of the organisms is done by collection of urine samples. The direct visualization of the organism is done under dark field microscope.
Serological techniques for diagnosis of Leptospirosis
Microscopic Agglutination Test (MAT)
Microscopic Agglutination Test (MAT) is considered the Gold Standard Test for detecting leptospirosis. This test is highly sensitive and is serovar/serogroup specific. MAT is therefore useful for epidemiological purpose since it gives clear idea regarding the circulating serovars in an endemic region. Ideally, MAT should be performed on paired sera collected during acute and convalescent stage of the disease to find out sero-conversion or four-fold rise in titre, which is the evidence of current or recent infection. Since collection of convalescent serum sample is difficult in routine practice, several disease investigators usually consider a titre of 1 in 100 as a significant titre of diagnosis without considering the endemicity or baseline titres in the community.
.
However MAT requires the use of several leptospiral serovars in their active growth phase whose maintenance is difficult, expensive, tedious and time consuming. The danger of acquiring the infection to lab technicians while handling the live leptospiral antigens, the cumbersome mechanisms of recording the results and the need for paired sera samples to confirm the disease which delays disease diagnosis has frustrated many researchers to adopt it as a routine test. Moreover MAT can give false negative test result when sera is collected from a patient in the early stages of the disease or when the patient is infected with a rare serovar which is not included in the test. MAT has also been reported to give false positive test result and low MAT titres can be due to cross reactive antibodies in patients suffering from Syphilis, Relapsing fever, Lyme disease, enteric fever, Dengue and Malaria which may give a titre of 1:80. MAT cannot distinguish between IgM antibodies indicative of current infection and IgG antibodies indicative of past infection. Further, MAT cannot differentiate between vaccinated and naturally infected animal sera and gives positive test result in both the cases which suggests that this test is not DIVA strategy based.
Latex Agglutination Test
Latex Agglutination Test for detection of antibodies to leptospires in serum samples is a rapid penside diagnostic test suitable for field conditions. This diagnostic test would not require the use of highly skilled labour and the test results would be very easy to interpret. The latex beads are sensitized with broadly reactive, specific antigen prepared from pathogenic leptospires. When the field sera is mixed with antigen coated latex beads, antibodies present in the sera interact with the antigen leading to the formation of fine and clearly visible granular agglutination. The intensity of the agglutination depends on concentration of the antibodies in a sera sample. Clearly visible granular agglutination indicates the presence of specific antibodies to leptospires. In stronger reactions due to sera of high antibody titre, fine granular clumps tend to settle at the edge of the circle and the reaction time to form fine granular clumps is lesser than 60 seconds. Agglutination that occurs beyond 2-3 minutes may be due to evaporation and should be treated as doubtful cases. The coated latex particles are stable for long periods at 4ºC and this long shelf life and cost effectiveness of this test makes this test a very desirable diagnostic tool for detecting leptospirosis. Unlike MAT which employs live whole Leptospiral antigens which pose danger to the lab technician performing the test, LAT employs only Outer Membrane Proteins of Leptospira.
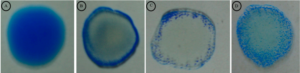
Latex Agglutination Test showing Leptospira –ve sera (A), +ve sera (B), ++ve sera (C) and +++ve sera (D)
(Figure courtesy of Leptospira Laboratory, Bacteriology Division, IVRI, Izatnagar)
Dot ELISA Dipstick Test (DEDT)
DEDT is a simple assay for the detection of Leptospira-specific IgM antibodies in serum samples. The assay is rapid and requires no special equipment. The ingredients are highly stable and can be stored at room temperature (20°C-25°C). The dipstick contains two horizontal bands: an antigen band consisting of broadly reactive Leptospira antigen (lower band) and an internal control (upper band). The assay is based on the binding of human Leptospira-specific IgM antibodies to the Leptospira antigen. Bound IgM antibodies are specifically detected with an anti-human IgM dye conjugate. The broadly reactive Leptospira antigen ensures the efficient detection of a wide spectrum of Leptospira infections. The assay is performed by making a 1:50 dilution of serum in the detection reagent and incubating a wetted dipstick in this solution. Staining of the antigen band reveals the presence of Leptospira-specific IgM antibodies in the serum sample. The internal control band provides a check on the integrity of the detection reagent and the presence of serum.

Dot ELISA Dipstick Test (DEDT) showing Leptospira +ve field sera (A-F), Positive control (G) and Negative control (H)
(Figure courtesy of Leptospira Laboratory, Bacteriology Division, IVRI, Izatnagar)
Enzyme Linked Immunosorbant Assay (ELISA)
ELISA is one of the techniques commonly used for the diagnosis of Leptospirosis. The test can detect specific IgM antibodies earlier than MAT. The advantage of the test is that it can differentiate between recent and past infection by detecting the type of antibodies (IgM or IgG) present in the sera sample. Anti-leptospiral IgM is detectable in this assay as early as 1 week after infection, before agglutinating antibodies are present. IgG antibodies are detectable in infected dogs beginning 2 weeks after infection and persist for long periods of time. Therefore, dogs with acute leptospirosis have high IgM titres and relatively low IgG titres, while dogs that are vaccinated or have had previous leptospiral infections have high IgG titres but low IgM titres. ELISAs have also been developed for use in milk from individual cows or in bulk tank milk for the detection of serovar Hardjo antibodies. These tests have been helpful in identifying Hardjo-infected herds. However, herds that are vaccinated against serovar Hardjo will also be positive in these ELISAs decreasing their usefulness in regions where vaccination is a routine practice.
rcPort Blair
Molecular tools in the diagnosis of Leptospires
Recently, DNA based techniques have been proposed as alternative methods of diagnosis and identification of leptospires. Nucleic acid probes and hybridization techniques were used previously for the detection of leptospires from clinical samples. Polymerase Chain Reaction (PCR) was subsequently developed for the detection of leptospires from clinical samples.
Polymerase chain reaction (PCR)
PCR involving the in vitro amplification of genus specific target DNA sequence of Leptospires has been developed to detect leptospiral infection in both animals and human beings. Van Eys et al developed PCR for detection of leptospires in urine samples of infected cattle. Urine samples containing as few as 10 leptospires per ml gave positive results in PCR assay. Gravekamp et al proposed the use of two sets of primers (G1 & G2 and B641 & B651) that enabled the amplification of target DNA fragments of various leptospiral species. Amplification with G1/G2 primers resulted in the generation of a PCR product of 285 bp length in all genospecies except L kirschneri whose genome can be detected by PCR employing B641/B651 primers which results in an amplicon of 563 bp length. These two sets of primers could detect even 1-10 leptospires per ml of urine. Leptospires were also detected by PCR from aqueous humor of patients with unilateral uveitis using silica particles and guanidine thiocyanate method. The advantage of PCR is its sensitivity, specificity and rapidity through which the disease can be diagnosed. However the major drawbacks of this technique are unavailability of facilities in common diagnostic laboratories and its high operational cost.
Hybridization with nucleic acid probes
Nucleic acid hybridization can confirm diagnosis before the results of culture is made available. Dot blot hybridization method with 32P and biotin labelled genotype specific probes has been used for early detection of leptospires. In situ hybridization method using biotin labelled DNA probe for detection of L interrogans in clinical samples has been done. However, hybridization with nucleic acid probes is tedious and costly and with PCR gaining more popularity the use of labelled probes for molecular diagnostics has diminished.
Treatment:
The antibiotic of choice for leptospirosis is Benzyl Pencillin which is administered as injection in doses of five million units per day for five days. Patients who are hypersensitive to Pencillin may be given Erythromycin 250mg four times daily for five days. Doxycycline 100mg twice daily for 10 days is also recommended. Tetracyclines are also effective but contraindicated in patients with renal insufficiency, in children and in pregnant women. Doxycycline has also been used as a chemoprophylactic agent for short-term exposure.
Prevention and control
Since rodents such as Rattus rattus and Rattus norvegicus are recognized carriers of leptospires, control at the carrier level by various rodent control measures play a pivotal role in curbing this disease. Inaccessibility of wild life at the animal farm is also important in disease control since leptospiral seropositivity has been observed in many wild animal species such as mongoose, oppossums and in wild rodents such as Shrews, Bandicoots and the aquatic rodent Coypu of France. Elimination of the reactors from cattle herd is essential but possible only in developed nations since many reactors become urinary shedders of leptospires. In developing countries owing to economic constraints, treating the reactors with suitable antibiotics in order to eliminate the shedder state becomes a practical approach to control the infection. Infected bulls should not be used for artificial insemination or natural service. In case of pigs the approach must be to treat all the animals in the herd than the individual pig.
Wearing of gumboots is essential in significantly reducing the incidence of leptospirosis in rice field farmers in southern Indian states with paddy fields especially Tamil Nadu and Andra Pradesh where people work with bare feet in wet environments. In Australia, Cane cutter’s fever/Sugar Cane worker’s disease as leptospirosis is known among sugarcane workers had been effectively controlled by wearing protective clothing which reduced micro abrasions of the skin through which the organisms gained entry. This success story can be replicated if similar measures are adopted by Central American sugarcane workers of El Salvador, Nicaragua, Costa Rica and Guatemala who have been dying of chronic kidney disease in which leptospirosis was suspected. Wearing of facemask to reduce exposure to aerosols and gloves should be practiced by people involved in high risk occupations such as butchers, pig Farmers, veterinarians and rodent control workers.
Vaccine
Inactivated leptospiral vaccines:
Although the only leptospirosis vaccine licensed for humans is being produced in Cuba, inactivated still acquire considerable interests. They are especially suitable as veterinary vaccines. When dogs were vaccinated twice with such vaccines, a high rate of protection against L. interrogans was observed and duration of immunity was at least 1-year. The efficiency of inactivated vaccine could be improved by adjuvant and vaccination frequency. A commercial inactivated bovine leptospirosis vaccine (with adjuvant) which induced a poor antibody production in cattle during preliminary vaccination showed a remarkable immune response after booster vaccination. Bovine leptospirosis vaccines available are pentavalent and contain leptospiral serovars pomona, grippotyphosa, canicola, icterohaemorrhagiae, and hardjo. These vaccines provide good protection against all serovars except serovar hardjo. The reason is that all bovine leptospiral vaccines contain the reference strain hardjo-prajitno. However cattle are infected with serovar hardjo-bovis. Thus the hardjo component of whole cell leptospiral vaccine becomes a weak immunogen.
Table. List of commercial leptospiral vaccines available for use in livestock and pet animals
| Name | Manufacturer | Serovars incorporated | Animal host |
| Nobivac | Intervet | Canicola | Dogs |
| Farrowsure | Pfizer animal health | Pomona, grippotyphosa, icterohaemorrhagiae, hardjo, canicola, porcine, parovirus, and eysipelas | Pigs
|
| Suileptovac TPC1 | Not known | Tarassovi, canicola, pomona, icterohemorrhagiae | Pigs |
| Leptoferm-5 | Pfizer animal health | Canicola, grippotyphosa, hardjo, pomona and icterohemorrhagiae | Pigs, cattle |
| Leptavoid H | Scherring-plough animal health, United Kingdom | Hardjoprajitno and hardjobovis | Cattle |
DNA Vaccines:
The low prices, easy administration properties and easy processing routes are some of the superior qualities which make DNA vaccine a promising new avenue to initiate further research. However, only two leptospiral DNA vaccine trials have been reported. DNA vaccine encoding endoflagellin flaB2 gene and hemolysis-associated protein 1 (Hap1) gene were tested in guinea pigs and gerbils respectively and partial protection against the infection by pathogenic strains of Leptospira was achieved.
Recombinant Protein based vaccines:
Only recombinant Proteins such as LigA/B, LipL41 and Hap1 proteins were approved as vaccines against Leptospira in animals. Loa22, the Koch Postulate antigen for leptospirosis is located in the outer membrane is an ideal candidate for a novel vaccine against leptospirosis. Many other recently reported outer membrane proteins (LAg42, Lk73.5), lipoproteins (LipL32, LipL45, LipL21 and GLP) and newly discovered virulence factors (Hsp58, FlaA, FlaB, SphH and ChpK) can help us to find more suitable vaccine candidates. Recombinant protein vaccines are usually stable, safe and free of contamination and easy to store and transport.

