Emerging Lumpy Skin Disease with its Diagnosis, Prevention and Control
Prachi Singh1*, Vikram Poonia2, Shubham Tiwari3 Anuradha Kaushik4, Puneet4
1MVSc Scholar, Department of Veterinary Microbiology, DUVASU, Mathura
2PhD Scholar, Department of Veterinary Parasitology, NDVSU, Jabalpur
3MVSc, Department of Animal Nutrition, ANDUAT, Ayodhya
4BVSc & AH, College of Veterinary Science and Animal Husbandry, DUVASU, Mathura
*Corresponding author-
Mobile number-8753921356
Email id- singhchinki97@gmail.com
Introduction-
Lumpy skin disease (LSD) is a notifiable non-zoonotic infectious disease restricted to ruminants viz. cattle and water buffaloes with high morbidity and low mortality rate. The causative agent of this disease is Lumpy skin disease virus (LSDV) of the family Poxviridae, subfamily Chordopoxvirniae and Capripoxvirus genus. It is known by several names such as “Neethling virus disease”, “Pseudo-urticaria”, “knopvelsiekte” and “exanthema nodularis bovis”. Due to significant economic losses and potential rapid spread- World Organization for Animal Health (OIE) has placed this transboundary disease on the notifiable disease list. It is the major threat to all ages and breeds of especially the young and lactation peak animals. The recent spread of this disease worldwide indicates the importance of understanding epidemiology, transmission, control as well as eradication for timely planning of effective disease management.
Emerging transboundary spread-
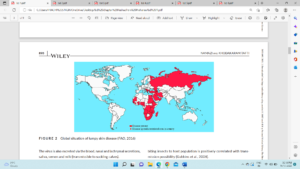
For the first time LSD virus was reported from Zambia in 1929 based on the abundance of biting insects as a case of hypersensitivity reaction for insect bites but it went unnoticed and then again reported in several regions of African countries in subsequent years. Due to the un restricted movement of infected cattle from affected African countries LSD infection was reported in Egypt in 1988, Turkey and Iraq in 2013 and Iran in 2014. Based on the OIE report, LSD has been re-emerged after 6 years in Israel in 2019 due to decrease in vaccination of animals. For the first time from 2010 to 2019, LSD outbreaks were reported from India, China and Bangladesh sharing boundaries with each distribution of number of outbreaks in Asian Countries. In India, first outbreak of the disease was reported in monsoon season in Odisha state in the month of August (2019) with high humidity and vector density. The reasons of the disease spread to India may be due to unrestricted livestock movement across international borders or vectors movement from the neighbouring countries. Based on the phylogenetic analysis, the LSD strain present in India during outbreak was genetically close to South African NI2490/KSGP-like strains rather than European strains.
Figure 1- Global lumpy skin disease outbreak situation (FAO, 2016)
Aetiology-
Lumpy skin disease virus (LSDV) is relatively larger in size (230–260 nm) comprising of an envelope and linear double-stranded DNA of 150 kilobase pairs (kbp) with 156 putative genes that replicates in cytoplasm of the host cell. The capsid is brick shaped with complex symmetry containing the genome and lateral bodies. The only serotype of this virus was first identified in South Africa “Neethling” and demonstrates similar antigenic properties with goat and sheep pox virus. As compared to other Chordopoxviruses, it has 146 conserved genes encoding proteins involved in nucleotide metabolism, structure formation, DNA replication, transcription, mRNA synthesis, virulence and host range. In the central region-65% genes share collinearity with other poxvirus genes particularly with suipox virus, leporipox virus, and yatapox virus. The terminal region shares difference of 43% average with either absence or disruption sharing lower percentage of amino acid identity. LSDV contains homologues genes such as G protein-coupled CC chemokine receptor (GPCR), interleukin-10 (IL10), IL-1 binding proteins, and epidermal growth factor-like protein which are found in other poxvirus genera.
The virus is susceptible to highly alkaline or acidic solutions- 20% chloroform, 1% formalin, ether, 2% phenol, 2–3% sodium hypochlorite, 0.5% quaternary ammonium compounds, iodine compounds dilution and the detergents containing lipid solvents detergents. This virus can be deactivated at at 55ºC for 2 hours, or 65°C for 30 minutes. It can even be recovered after 10 years from the skin nodules kept at -80ºC and if kept at 4ºC after 6 months from the infected tissue culture fluid.
Transmission –
In order to study the epidemiology of the virus it is important to study the mechanism of LSDV transmission that can be utilized as a criterion for prevention and disease extinction.
Vector transmission-
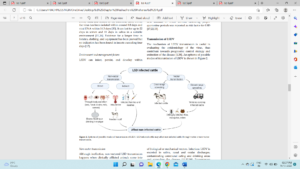
The LSDV is transmitted through arthropods, particularly blood-sucking insects, mosquito aedes particularly, ticks and flies. With the onset of winters, incidences decrease significantly and reappears with arrival of spring and summer. In the tick host, it can be transmitted trans-stadially and trans-ovarially during cold temperatures. It can spread in short distances of a few kilometers and even longer-distance due to unrestricted animal movements across international borders.
Non-vector transmission-
As the virus remains in the lesions or scabs for long periods, it acts as the main sources of infection. It is also excreted via the saliva, nasal, blood, and lachrymal secretions, semen and milk (transmissible to suckling calves). Iatrogenic route can be also act as a route of spread when single needle used for mass vaccination.
Figure 2- Mode of LSD virus transmission (Das et al., 2021).
Risk factors-
They can be classified under three categories-
Host associated factors-
- Severely affect the cattle and Asian water buffalos where buffaloes have lower morbidity rate.
- Indigenous (Bos indicus) breeds are less vulnerable to clinical disease
- Immunocompromised animals are more susceptible to the disease condition.
- young animals exhibit higher susceptibility and severity than the aged cattle
- Wildlife act as the possible viral reservoir
- Giraffe (Giraffa camelopardalis) and impala (Aepyceros melampus) showed susceptibility to LSDV in experimental inoculations.
Agent related factors
The virus remains viable in:
- Cattle blood-8.8 days
- Virus DNA-16.3 days
- Semen-22 days
- Saliva-11 days
- Existence in fomites, clothing, and equipment is evident for a longer time but no records were found in insects exceeding four days.
- Other factors like herd size, vector populations, migration of herd, transport of infected animals into disease-free areas, common pasture and water sources may increase the disease prevalence.
Environment and management factors
- Warm and humid conditions favour higher proliferation of mosquitoes, flies, and ticks.
- Higher morbidity in intensive large farms compared to the backyard small farms were reported in few studies.
- Common watering and grazing points facilitate virus circulation that facilitates virus transmission.
Pathogenesis-
The spread of the virus occurs after the early febrile condition through blood and form generalized lymphadenitis followed by viremia for almost 4 days. Formation of multiple circumscribed cutaneous nodules following LSD virus infection is characteristic finding of this disease. Intracellular replication occurs in fibroblasts, macrophages, pericytes and endothelial cells leading to vasculitis and lymphangitis in affected tissues. Animals recovered from natural infection by the virus have shown lifelong immunity.
Clinical signs-
Incubation period of this disease lies between 2-5 weeks. This disease is characterised by slightly raised firm circumscribed skin nodules of 2-7cm diameter on the neck, legs, tail and back region. It occurs in three forms: acute, subacute and chronic form. The illness begins with biphasic fever.
- Mild form is characterized by- one or two nodules within 2 to 3 days of fever. Later on they become painful and hyperaemic in the skin of various region.
- Severe condition is characterised by more than hundred nodules developed all over the body skin and this stage persist for 7 to 12 days. The lesions will then progress to papules, vesicles, pustule and slowly to scab formation. Healing of the lesions is very slow. After few days, sloughing of the lesions may create hole forming “sit fast” characteristic lesion facilitating the invasion by fly and bacteria that can further lead to septicaemia.
Sequelae-
- Due to the inhalation of necrotic material- pneumonia
- Abortion in acute phase infection.
- Infertility in both male and female.
- Persistent anoestrus condition in females
- Secondary bacterial infection, mastitis and fly strike in necrotic lesions leaving deep holes in the body
- Complications of severe disease includes- keratitis, dysentery, lameness, pneumonia, mastitis and myiasis.
Histopathological findings-
Pathognomonic finding includes- eosinophilic intracytoplasmic inclusion bodies in the endothelial cells, keratinocytes, macrophages and pericytes associated with the ballooning degeneration of spinosum cells. Surrounding tissue of epidermis, dermis, and core musculature reveal haemorrhages, congestion, and oedema with distended lymph nodes.
Economic and social impact-
The socio-economic impact of LSD can be direct or indirect based upon several major sectors and industries.
Direct impact includes-
- Sharp decline in milk production that causes a considerable reduction in milk yield (from 10% to 85%) due to high fever and secondary mastitis.
- Despite the possibility of the meat having secondary bacterial infection from LSD infected cattle is not prohibited from entering the food chain.
- Damaged hides, temporary or permanent infertility, abortion.
- Treatment and vaccination costs and death of infected animals.
Indirect impact includes-
- Trade restriction, quarantine, immunization, treatment costs, feed and labor costs, maintenance of farm biosecurity, etc.
Total production losses have been estimated at 45%–65% resulting from the disease in industrial cattle farming. India with the global exporting position of ninth earns annual revenue of US$ 8,500 million for its leather and leather products.
Diagnosis-
The diagnosis of this diseases is challenging due to lack of logistics. To make a presumptive LSD diagnosis- clinical history, signs and symptoms of infected animals can be used. Samples should be carried in 20-50% glycerol phosphate buffer saline transport medium. Skin samples can be visualized using electron microscopy. Despite primary clinical diagnosis confirmatory diagnosis is done by conventional PCR or real-time PCR techniques that differentiates LSDV from sheep and goat. In new niches virus isolation can be used for the confirmatory diagnosis. The bovine testes and pre-pubertal lamb are most sensitive for isolation. For differentiating between virulent LSDV and vaccine strain, Restriction Fragment Length Polymorphism (RFLP) has also been developed. From clinically infested animals, fluids like saliva, nasal swab, or whole blood can be collected for viral isolation and molecular testing.
Additionally, the disease can be detected by using serological tests i.e., Indirect Fluorescent Antibody test (IFAT), Enzyme-linked Immunosorbent Assay (ELISA), Indirect Immunofluorescence test, Virus Neutralization Test (VNT) and Serum Neutralization Test (SNT). A fairly new assay with the potential of LSD diagnosis i.e., Immuno-peroxidase Monolayer Assay (IPMA) has been developed that is economically feasible as it is adapted to low biosafety levels with higher sensitivity and specificity than VNT and commercial ELISA.
Prevention and control-
Treatment of LSD is only symptomatic that targets at preventing secondary bacterial infections by using a combination of supportive therapy by antimicrobials, anti-inflammatory, and anti-septic solutions.in order to control the disease, effective control and preventive measures need to be implemented, which include:
- a) Restrict vector movements: By the use of vector traps, insecticides for preventing the disease.
- b) Restrict movement: Movement of infected animals should be strictly prohibited to prevent the spread of transboundary disease. If animal with LSD lesions are observed within countries, rapid spread of disease is controlled by proper quarantine.
- c) Vaccination:
- Homologous (Neethling strain)- passaged 60 times in lamb kidney cells and 20 times on the chorio-allantoic membrane of embryonated chicken eggs
- Heterologous live attenuated vaccine (Sheep/Goat pox vaccine)- best medical prophylaxis for LSD. These vaccines cause local reactions thus not advised in sheep pox and goat pox affected areas.
- Sheep pox vaccines used against LSD includes-
- Kenyan sheep pox virus- passaged 18 times in lamb testis (LT) cells or fetal calf muscle cells,
- Yugoslavian RM 65 sheep pox strain,
- Romanian sheep pox strain.
- Gorgan goat pox strain- provide good protection in cattle with practically no side effect
In India, only after the first documented LSD outbreak in 2019, heterologous live attenuated GTPV (Uttarkashi strain) vaccine has been officially permitted for emergency use in cattle and buffaloes. Also, ICAR-NRCE, Hisar in collaboration with IVRI has developed homologous live attenuated LSD vaccines commercially available as- “LumpiProVacInd”.
Long term vaccination with 100% coverage should be made mandatory as LSD virus for effective control and prevention of disease. Pregnant cows, breeding bulls can be vaccinated annually. The animals should be immunized before introducing new animals to the affected farm at the age of 3 to 4 months raised from mothers, who are vaccinated or infected naturally.
Conclusions-
Earlier the disease was restricted to African and few other countries but the recent spread of disease to India and other Asian countries, is subject of concern for the livestock rearing sector as most of these countries have agriculture-based economies. The recent outbreak of this disease indicates its economic and epidemiological significance. Therefore, accurate and timely diagnosis in endemic areas, vector control, animal movement restriction vaccination with the homologous strain of the LSDV, and its testing of bulls used for breeding are highly recommended as tools to control further spread. Thus, it is the high time to practice preparedness to limit the spread of this trans-boundary disease enormously.
References-
Das, Moumita & Rahman Chowdhury, Md. Shahidur & Akter, Sharmin & Mondal, Apurbo & Uddin, Md Jamal & Rahman, Md & Rahman, Md. Mahfujur. (2021). An updated review on lumpy skin disease: perspective of Southeast Asian countries. 10.5455/jabet.2021.d133.
FAO. (2016). Food and Agriculture Organization of the United Nations, Global animal disease intelligence report – Annual Issue NO. 5.
Gupta T, Patial V, Bali D, Angaria S, Sharma M, Chahota R. 2020. A review: Lumpy skin disease and its emergence in India. Vet Res Commun, 44(3-4):111 118.
Koirala P, Meki IK, Maharjan M, Settypalli BK, Manandhar S, Yadav SK, Cattoli G, Lamien CE. 2022. Molecular Characterization of the 2020 Outbreak of Lumpy Skin Disease in Nepal. Microorganisms, 10(3):539.
Namazi F, Khodakaram Tafti A. 2021. Lumpy skin disease, an emerging transboundary viral disease: A review. Vet Med Sci, 7(3):888-896.