How to get Veterinary Drug License for Veterinary Medicine Shop in India
Drug license is a permit granted by the government under THE DRUGS AND COSMETICS ACT, 1940 to deal with drugs. In India, Drug for Humans & Animals are regulated with the same acts & regulations ie. THE DRUGS AND COSMETICS ACT, 1940 and Rules, 1945 . There are two kinds of licenses namely retail license and wholesale license that is mandatory for drug distribution or sale in India. The license is issued after the verification of certain requirements pertaining to the premises and the person dealing with drugs.
Drug Regulation
Drug Regulation is a system to monitor and protect the public health and animal, by controlling the safety and efficacy of the products in areas like pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines.
Regulatory Process for Approval of a New Drug
An application to conduct clinical traits in India has to be submitted along with the data of chemistry, animal studies to DCGI and to the manufacturing control. The date regarding the trial protocol, informed consent documents and investigator’s brochures have to be attached. A copy of the application should be submitted to the Ethical Committee. Only after the approval of DCGI and the Ethical Committee, the clinical trials are conducted.
Features of Drug License
The basic features of a drug license are listed below:
- Mandatory for drugs and cosmetics business.
- Drug license is granted in commercial premises for the sale of medicines and cosmetics.
- Pharmacy business has to abide by all the conditions of the license.
- Drug license is displayed at a noticeable place of the premises.
Classification of Drug License
Drug licenses are classified into three categories. They are
- Manufacturing License
- Sale License
- Loan License
Manufacturing License
Manufacturing license is required for manufacturing allopathic/ Homeopathic medicines and cosmetics and to repack drugs. This license is issued only in recognized industrial areas.
Sale License
Sale license is required to sell drugs to the customers. This license is further classified as
- Retail Drug License
- Wholesale Drug License
Loan License
Loan license is issued to an individual who intends to avail the manufacturing facilities owned by another licensee. If a pharmacy business is run in more than two states, then this license has to be obtained in every place where the business is carried on.
Infrastructure Requirements
To establish a medical shop or a pharmacy, a minimum of 10 square meters is required. Store business combination with retail and wholesale, a minimum of 15 square meters is required.
Storage Requirements
It is mandatory to have a refrigerator and an air conditioner on the premises because certain drugs such as vaccines, insulin injections, etc have to be stored in the fridge.
Technical Staff
Online Sale: Online sale of a drug is possible only when there is a registered pharmacist is present who is approved by the concerned department. The pharmacist has to be present throughout the working hours in the retail shop.
Wholesale: Wholesale of drugs can be made either by a registered pharmacist or by another person who has to be a graduate with one year experience in drugs or the person has to pass S.S.L.C with four years experience in drugs who is specially approved by the drug control department.
Types of Drug License in India
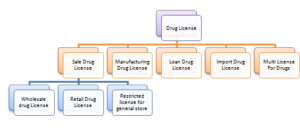
Drug License in India must be acquired by all those persons who are engaging in the business of the pharmaceutical industry. The classification of Drug license will depend on the categories of drugs and the business involved with them. Respective State Government will issue this License according to their eligibility terms.
In this article, we will discuss various types of Drug License in India and how to acquire this License.
What is Drug License?
Drug License in India is a kind of permission by the concerned State Authority to the manufacturers, retailers, wholesalers, distributors and all those who are engaging in the business of pharmaceuticals for carrying out the business activities related to the pharmaceutical industry.
Respective State Drug License Authority issues this License under the Drugs and Cosmetics Act, 1940. There are three significant classifications of Drug License in India which may be further divided into further categories.
What are the different types of Drug license in India?
Manufacturing Drug License in License
This License must be acquired by the manufacturers of Allopathic, Ayurvedic, Cosmetics products or other drugs under the Drugs and Cosmetics Act, 1940. The respective State Government grants this License to the applicant.
Loan Drug License in India
Loan Drug License is issued to those authorities who do not have their own land for manufacturing their drugs but want to manufacture the products and services with their brand name on land of those are already issued with the License of manufacturing.
Import Drug License
This Drug License must be acquired by those persons who are using imported products for manufacturing drugs or those who are engaging in the distribution of imported drugs in India.
Multi License for Drugs
Multi-Drug License must be acquired by those entrepreneurs who are operating in more than one state. No one can sell drugs at more than one place without obtaining this Multi-Drug License.
Sale Drug License in India
The Sale Drug license in India is acquired by wholesalers and retailers who are engaging in the distribution of drugs in India. There are three different types of Drug License in India as follows:
Wholesale Drug License in India
The wholesale Drug License in India is issued by CDSCO (Central Drugs Standard Control Organization) to those wholesalers who are doing business in pharmaceuticals. The person who wants to obtain this License must have some degree or diploma in pharmacy from a recognized university and a minimum of one year experience in drug dealing. To get this License, the applicant must submit these following additional documents:
- Form-19
- A letter stating the purpose of this License
- Challan of fees
- Receipt of Property Tax
Retail Drug License
Retail Drug License in India is issued by Licensing Authorities to retailers who are doing business in pharmaceuticals, stand-alone pharmacists, etc. The applicant should be registered pharmacist under the State Pharmacy Council.
Restricted License for General Store in India
This License is issued under Forms 20A and 21A to dealers or persons, to sell drugs without the supervision of any qualified person.
What are the essential requirements for obtaining Drug License in India?
Area for the operations of the business
A minimum area of 10 sq meters is required to set up a medical shop or retail pharmacy. If they are operating with a wholesale one, and then the minimum of 15 square meters is mandatory.
Proper Storage Facility
There are several medicines and vaccines which need to be restored at cool places, so the requirement of A/C and refrigerators is necessary for the storage.
The requirement of technical staff
The presence of a qualified and experienced person is necessary to operate a retail pharmacy in India.
What are the documents required for obtaining the Drugs License in India?
- Passport size photograph of the owner(s)
- Identity proof of owners
- Address proof of owners
- Evidence for depositing the application fees
- Application form, Filled and signed
- Self-attested copy of the registered plant layout
- Utility bill as the certified office proof, in case of owned land
- Rent agreement, if applicable
- No-objection Certificate from Landlord
- Educational background, experience letter of the registered pharmacist
- Invoice of Refrigerator for business purpose
- Invoice of the purchase of Air-Conditioner
Additional documents required in case of partnership firm
- Partnership deed
- Complete list of partners
Other documents required in case of Private and Public Limited Company
- Certificate of Incorporation
- Memorandum of Association
- Article of Association
- Appointment of the concerned and authorized person who will be responsible for daily operations.
- Copy of the board resolution regarding the authorized person who will be responsible for the day to day operations.
Additional documents required in case of a pharmacist
- Copy for the diploma in pharmacy
- Registration Certificate from The Pharmacy Council
- Experience Letter, if any
- Affidavit of pharmacist
- Details of the educational certificate of a competent person
What is the procedure for obtaining Drug License in India?
Step-1 Signing up on Website
Firstly, the applicants need to visit the website of their respective State Drug Licensing authority and register themselves by filling the registration purpose.
After that, the applicant has to fill his details, organization details, and contact details to generate OTP.
Step-2 Uploading documents
After filling up the form, the applicant has to upload documents; the documents checklist is already mentioned earlier depending on the formation of the Company.
Step-3 Payment of Fees
After uploading all the documents, requisite fees of the form should be submitting and generate challan of that for further processing.
Step-4 Securitization of Documents
After submitting the form, the licensing authority will scrutinize the form, and the DOC (District Coordination Officer) of the concerned district will inspect the organization and ensures that it comply with all the requirements.
After the verification and inspection, the DCO will forward the report to the SDCO of the zone for granting the License.
Step-5 Issuing Drug License
The report of SDCO will be forwarded to the respective State Drug License Authority, and, if the authority finds no discrepancy in the report, then it will issue Drug License to that person. Generally, it will take a minimum of 30 days for the issuance of a Drug license in India, if the applicant fulfilled all the requirements.
What is the validity of a Drug License in India?
Usually, the validity of the Drug License in India is five years.
Importing Drug in India
Requirements to import rugs in India as specified in Schedule X and those drugs that are not specified in schedule require an Import License. If more than one drug is manufactured by the same manufacturer, a single license may be issued. A manufacturer having more than two factories manufacturing drugs in different places, a separate license will be required for each factory.
Application Procedure
The application procedure for the grant of selling license is given below.
Step 1: Obtain the Application
The applicant has to fill the required Forms 19, 19A,19B, 19AA.
Step 2: Fee Payment
The applicant has to remit the required fee.
Step 3: Inspection
The concerned officials survey the application and inspect the premises.
Step 4: Grant of License
If all the requirements have complied, the authority issues the license.
Documents Required
- Duly filled Application Form.
- Fee receipt or challan, as the case may be or their attested copies.
- 3 copies of the layout of the selling premises.
- Documents like rent receipt, purchase documents or its attested copies that mentions the lawful possession of the premises.
- Documents pertaining to the constitution of the firm like Memorandum, Partnership-deed, and article of association etc.
- Details of the competent technical staff / registered people with copies of experience, educational qualification and registration certificates.
- Full name of the partners or the proprietors, as the case may be has to be mentioned in the application. For private or public limited concerns, the full name of the Directors who signs the application and the authorised signatory, if any, has to be provided in the application form.
- Documents for the purchase of Deep freezer/ Refrigerator (For Vaccines / Sera).
Renewal of Drug License
The procedure for renewing the sale of drug license is given below.
Step 1: Obtain the Application
The applicant has to fill the required Forms 19, 19A, 19AA, 19B, 19C.
Step 2: Fee Payment
The applicant has to remit the required fee.
Step 3: Inspection
The concerned officials survey the application and inspect the premises.
Step 4: Grant of License
If all the requirements have complied, the authority issues the license.
Documents Required
The following are the documents required to renew the license required for selling drugs.
- Copy of cash receipt or challan for payment of fees.
- Xerox copy of Licence or the previous renewal certificate.
- Documents such as the rent receipt, purchase documents or its attested copies showing lawful possession of the premises.
- Details of the competent technical staff/ registered people along with copies of their educational qualification, experience and registration certificates.
Conclusion
- Therefore, it is quite challenging to obtain or to register for Drug License in India. So, it would be a better idea to hire professions for your assistance in obtaining Drug License in India for your business.

