Role of Zinc in Animal Nutrition
Praveen Kumar Agrawal1, Bharti Yadav2 Monika Karnani3, Sheela Choudhary4, and Manju5
Department of Animal Nutrition
Post Graduate Institute of Veterinary Education and Research (PGIVER), Jaipur
1 PG Scholar, Department Of Animal Nutrition, PGIVER, Jaipur
2PG Scholar, Department Of Animal Nutrition, PGIVER, Jaipur
3Assistant Professor, Department Of Animal Nutrition, PGIVER, Jaipur
4 Professor& Head, Department Of Animal Nutrition, PGIVER, Jaipur
5 Assistant Professor, Department Of Animal Nutrition, PGIVER, Jaipur
Zinc is an essential nutrient for animals functioning largely or entirely in enzyme system and being involve in protein synthesis, carbohydrate metabolism and many other biochemical reactions. A severe deficiency of zinc causes numerous pathological changes including parakeratosis reduced or ceased growth, debility, lethargy, infections susceptibility. After the discovery that zinc was essential for growth and health in rats (Todd et al., 1934) and present in an enzyme, carbonic anhydrase (CA) (Keilin and Mann, 1940), found in blood, studies were made in livestock.
Significance of Zinc (Zn)
Zinc (Zn) is the second most abundant trace element in the animal body. It can’t be stored in the body and requires regular dietary intake to meet the physiological needs. Zinc is necessary for the proper physiological functioning of phosphatase (ALP), aldolase, lactate de-hydrogenase (LDH), RNA and DNA polymerases, reverse transcriptase, carboxypeptidase A, B, G and superoxide dismutase (SOD).
Functions of Zinc (Zn)
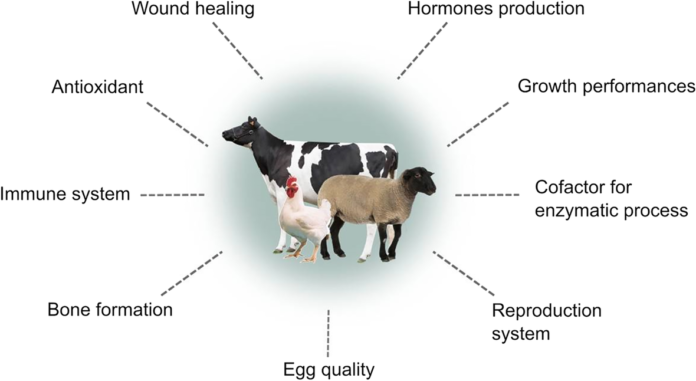
Zinc is arguably the most influential of minerals, known to catalyse, co-catalyse or provide structural integrity to around 300 enzymes in all major categories, from hydrolases to transferases (Choi et al., 2018): ten times as many transcription factors require Zn for structural integrity (King et al., 2016). Tetrahedral co-ordination of Zn to cysteine and histidine residues creates ‘zinc-finger’ domains that stabilize protein folds (Berg, 1990). The long-established function of zinc as catalyst of angiotensin-converting enzyme 2 (ACE 2) has a broader facet ACE 2 hydrolyses other peptides, including some involved in humoral and innate immunity (Bernstein et al., 2018). Experimentally discovered function of zinc emerged from studies from rats & mice showing zinc effect on appetite sensitivity and expression as zinc deprivation causes expression of CCK (cholecystokinin)- appetite regulating hormone and leptin (satiety signal) are increased.
Zinc insufficiency affect membrane signalling system and intracellular messenger that coordinate cell proliferation in response to IGF-I (insulin dependent growth factor). (McDonald 2000).
Zinc act as a pro- antioxidants providing structural stability to a superoxide dismutase (CuZnSoD) that protect cell from oxidative damage by oxide free radicals by inhibiting lipid peroxidation (Zago&oteiza2001) & enhances defensive mechanism by inducing thionenin (MT)- a zinc and thiol rich protein that act as redox sensor in cells (Bealtie & trayhum 2002).
Zinc affects foetal growth reflecting role in DNA synthesis and nucleic acid metabolism .Zn is believed to regulate virtually all aspect of innate and adaptive immunity via a network of transport protein (Zn T &ZIP) (Haase and Rink, 2014).
Zn influence remodelling of trabecular bone by controlling PH in presence of Ca in osteoblast
Natural zinc sources
World widely Zn in pasture ranged from 7 to >100 but most lay between 25 and 50mg kg−1DM, influenced by soil Zn and sward maturity (Minson, 1990). . Hays tend to contain less Zn than silages, concentrations varying little between grass species, although Rhodes grass is often low in Zn (e.g. Jumba et al., 1995). Legume species can vary widely, irrespective of soil Zn (Minson, 1990). Cereal (grain) Zn varies little among species and values are influenced more by soil Zn status Cereal Zn is concentrated in outer layers of the grain in association with phytate. The degradability of phytate determines the nutritive value in phytate-rich feeds. Cereal straws usually contain only a third of the concentration found in the grain. Vegetable and animal protein sources are richer but more variable in Zn than cereals. Normal cow’s milk contains 3–5 mg kg−1DM Zn and colostrum far more (14 mg kg−1DM) mostly associated with α2 casein. The large amounts of Zn secreted after birth by cows may contribute to the temporary fall in plasma Zn around parturition (Goff and Stabel, 1990). Supplementation of the mother will not increase milk Zn unless the diet lacks Zn (see Miller, 1970; Schwarz and Kirchgessner, 1975). Both dried skimmed milk and buttermilk are therefore good sources, commonly containing between 30 and 40 mg Zn kg−1DM.
Nano Zinc, an Alternative to Conventional Zinc as Animal Feed Supplement
Zn from conventional sources is less available to the body and thus mostly excreted to the environment causing environmental pollution. Nano Zn, as a substitute to the conventional Zn sources, can be a good alternative in livestock feeding. Apart from being highly bioavailable, reports have pointed out the growth promoting, antibacterial, and immuno-modulatory and many other beneficial effects of nZn. This also serves all the purposes of the conventional Zn sources and helps in all the physiological functions. Thus, Nano Zn may be used at lower doses in livestock feed to provide better results than the conventional Zn sources and indirectly prevents environmental contamination also. The toxicological studies provide mixed results in animal models. So, thorough and systematic studies are recommended for elucidating toxic effects, in any, dose fixation and also for economic production procedures to take nZn journey to logical conclusions.
Availability of Zn in Feeds
Zinc is absorbed according to need by an active saturable process in apical mucosal cells from the duodenum to the colon (Martin et al., 2013a) and involves a series of ZnT and Zip proteins Efficiency of absorption (AZn) declines exponentially as dietary Zn increases and potential absorbability (AZn) can only be measured with dietary Zn close to requirement Apparent absorption (AAZn) decreased from 0.47 to 0.22 as dietary Zn increased from 40 to 1000 mg kg−1DM in a milk replacer for calves (Jenkins and Hidirolglou, 1991). Apparent absorption (AAZn) decreased from 0.47 to 0.22 as dietary Zn increased from 40 to 1000 mg kg−1DM in a milk replacer for calves (Jenkins and Hidirolglou, 1991). In monogastric species, antagonism of AZn by phytate is a major determinant of availability and inclusion rates for dietary components that influence that antagonism affect the recorded AZn or AAZn. Relative biological values (RBVZn) are also subject to dietary influences. . Using basal diets low in phytate underestimated RBVZn in phytate rich feeds. Brans rich in phytase improve AAZn when included in the diet (Roberson et al., 2005) but efficacy will not be fully expressed if zinc is provided in excess of requirements.
Dietary Zinc Requirements
Grazing cattle or sheep on pasture have good Zinc concentration <20Mgkg\DM & doesn’t feed any additional supplementation. In poultry chicks (starter) indicated a need to add 5–10 mg Zn to a corn/SBM blend containing 23 mg inherent Zn kg–1DM (Ao et al., 2006). Hatchlings from eggs laid by hens deprived of Zn required >50mg Zn kg−1DM to prevent feather fraying. In experimentation on chicks and piglets there is lower need in chicks than in piglets was confirmed by feeding both species on the same diet and attributed to a low pH in gizzard and stomach, which promoted phytate degradation and zinc solubilization (Schlegel et al., 2010). The old recommendation of 65mg Zn kg−1DM for egg production (NRC, 1994) was confirmed but more (70–80 mg) recommended to optimize tibia Zn and plasma. Breeder hens have similar needs for egg production to layers but higher needs for optimum fertility. The higher requirement for laying and breeding than growing birds is partly due to a high net requirement for each egg produced and partly to the strong antagonism from phytate on diets rich in Ca throughout lay (>30g kg–1DM). In the transition to lay, zinc provision for pullets should increase in line with calcium provision from 25 to 70mg kg–1DM. Requirements for optimal wool growth, plasma Zn and male fertility (14mg Zn kg−1DM). Growth rate and FCE were improved in cashmere goats by raising dietary Zn from 22mg Zn kg−1DM.
Deficiency
Zinc is an essential component of many enzyme systems, including those related to protein and carbohydrate metabolism, and is essential for maintaining a healthy coat and skin. Zinc is normally absorbed from the intestine at approximately 20-30% efficiency and competes with copper, iron and calcium for absorption. Zinc deficiency may occur because of inadequate intake or availability, malabsorption or increased rates of loss from the organism. Decreased zinc availability has been seen in certain dog foods containing excessive levels of phytate which inteferes with zinc absorption. Zinc deficiency is mainly seen in dogs and pigs, sometimes in ruminants and horses.
Pigs present with a disease called parakeratosis which appears as circumscribed reddened papules and plaques, thick crusting and scaling, fissures along ventral abdomen and medial thighs. Sometimes the condition is generalised and pigs may show mild lethargy, anorexia and depression. The skin lesions may resemble those caused by Staphylococcus hyicus however this usually occurs in younger piglets.
There is commonly secondary bacterial dermatitis which can complicate the diagnosis.
In dogs, a marginal deficiency may result solely in skin changes which are very noticeable to the owner. Zinc-responsive dermatosis is commonly seen in Siberian Huskies and Alaskan Malamutes and is characterised by scales and crusts around mouth, chin, eyes, joints, prepuce, scrotum and vulva. In rapidly growing puppies, there will be scaly plaques on the skin, nasal planum and foot pads. Canine zinc deficiency has also been described as producing ocular signs of mucopurulent exudation, blepharitis and keratitis. Severe zinc deficiency will present as poor growth, anorexia, testicular atrophy and emaciation. In ruminants, there is alopecia, crusts and scales on face, neck and distal extremities and mucocutaneous junctions. Low zinc status leads to lower quality milk and increased incidence of mastitis in foals, zinc deficiency causes reduced growth rate, anorexia, cutaneous lesions on the lower extremities and alopecia. In poultry swollen hock syndrome.
Zn Toxicity
Simple-stomached livestock are extremely tolerant of high Zn intakes, tolerance depending partly on species but mainly on dietary factors that limit availability, like phytate and calcium Weanling pigs can consume diets containing 2–3g Zn kg−1DM for several weeks but growth and appetite are depressed at 4–8g kg−1DM and mortality is high (NRC, 2005) Broilers and layer hens tolerated 1–2g Zn kg−1DM and showed only slight growth and appetite depression at 4g Zn kg−1DM (Oh et al., 1979). Laying hens were fed diets with 20g Zn kg−1DM for 14 days to reduce food intake and induce moult, without adverse long-term consequences (Stevenson and Jackson, 1984): high Ca intakes of laying birds may have been protective. Weaned ruminants are also relatively intolerant of Zn, probably due to lack of protection from phytate and vulnerability of rumen microflora: ≥1.7g Zn kg−1DM induced pica and disrupted rumen fermentation in lambs (Ott et al, 1966). For diagnosis, high Zn in feed, digesta and faeces (i.e. g >mg kg−1DM concentrations) are indicative of toxicity.
Interrelation with Other Element
In monogastric animals it is well established that Ca, Cd, Mg, P, Cu chelating agent (EDTA, Vitamin D, Phytic) influence Zn absorption & metabolism at cellular level most major example of it was parakerotosis in swine when occure due to reduce Zn absorption from diet which contain plant protein, soyabean meal & high Ca level.
Conclusion
Zinc is needed in many enzymatic and metabolic function in avian and mammalian species.it participates in antioxidant and defence mechanism of body. Zinc deficiency affects detrimentally to animal growth and production along with embryonic anamolies in chicks and sometimes mortality is seen in severe cases.
Reference:
Batal, A.B., Parr, T.M. and Baker, D.H. (2001) Zinc bioavailability in tetrabasic zinc chloride and the zinc requirement of young chicks fed a soya concentrate diet. Poultry Science 80, 87–90.
Beattie, J.H. and Trayhurn, P. (2002) Metallothioneins and oxidative stress. Nutritional Sciences 5, 228–233.
Bernstein, K.E., Khan, Z., Giani, J.F., Cao, D.Y., Bernstein, E.A. and Shen. X.Z. (2018) Angiotensin converting enzyme in innate and adaptive immunity. Nature Reviews in Nephrology 14, 325–336.
Berg, J.M. (1990) Zinc fingers and other metal-binding domains: elements for interactions between molecules. Journal of Biological Chemistry 265, 6513–6516.
Goff, J.P. and Stabel, J.R. (1990) Decreased plasma retinol α-tocopherol and zinc concentration during the periparturient period: effect of milk fever. Journal of Dairy Science 73, 3195–3199.
Jenkins, K.J. and Hidiriglou, M. (1991) Tolerance of the pre-ruminant calf to excess manganese and zinc in a milk replacer. Journal of Dairy Science 74, 1047–1053.
Jumba, I.O., Suttle, N.F., Hunter, E.A. and Wandiga, S.O. (1995) Effects of soil origin and mineral composition and herbage species on the mineral composition of forages in the Mount Elgon region of Kenya. 2. Trace elements. Tropical Grasslands 29, 47–52.
Keilin, D. and Mann, T. (1940) Carbonic anhydrase: purification and nature of the enzyme. Biological Journal 34, 1163–1176.
Kennedy, K.J., Rains, T.M. and Shay, N.F. (1998) Zinc deficiency changes preferred macronutrient intake in subpopulations of Sprague-Dawley outbred rats and reduces hepatic pyruvate kinase gene expression. Journal of Nutrition 128, 43–49.
King, J.C., Brown, K.H., Gibson, R.S., Krebs, N.N., Lowe, N.M., Stekmann, J.H. and Raiten, D.J. (2016) Biomarkers of nutrition for development (BOND)-Zinc review. Journal of Nutrition 146 (Suppl), 858S–85S.
McDonald, R.S. (2000) the role of zinc in growth and cell proliferation. Journal of Nutrition 130, 1500–1508S.
Miller, W.J. (1970) Zinc nutrition of cattle: a review. Journal of Nutrition 53, 1123–1135.
Minson, D.J. (1990) Forages in Ruminant Nutrition. Academic Press, San Diego, California, pp. 208–229.
Oh, S.H., Nakane, H., Deagan, J.T., Whanger, P.D. and Arscott, G.H. (1979) Accumulation and depletion of zinc in chick tissue metallothioneins. Journal of Nutrition 109, 1720–1729.
Roberson, K.D., Kalbfleish, J.L., Pan, W., Applegate, T.J. and Rosenstein, D.S. (2005) Comparison of wheat bran phytase and a commercially available phytase on turkey tom performance and litter phosphorus content. International Journal of Poultry Science 4, 244–249.
Todd, W.R., Elvehjem. C.A. and Hart, E.B. (1934) Zinc in the nutrition of the rat. American Journal of Physiology 107, 146–156.