Futuristic Approaches to Triumph Over Antimicrobial Resistance: A Need of the Hour
Authors: Dr. Debasish Behera1* and Dr. Ashabaree Samal2
Authors affiliation
1* Assistant Professor, Department of Veterinary Pathology,
C.V.Sc and A.H, R.K. Nagar, West, Tripura-799008
2Assistant Professor, Department of Veterinary Microbiology,
C.V.Sc and A.H., OUAT, Bhubaneswar-751003
Abstract
The action of antimicrobial substances is to inhibit the growth of microorganisms by the way of lack of metabolic process and by nonappearance of binding or target site. But, the acquired antibiotic resistance is achieved broadly via two ways i.e. chromosomal mutation and biochemical mechanism. The antimicrobial resistance of bacteria reveals that, the genes are mainly responsible and the plasmid associated genes play the critical role. AMR is acquired by the pathogenic bacteria by showing resistance in terms of prevention of bacterial protein synthesis, disruption of cell membrane function and inhibition of bacterial cell wall synthesis. The global use and misuse of antibiotics has led to the evolution and spread of bacterial resistance to all regularly used antibiotics. Therefore, the novel and futuristic approach to tackle the AMR, by developing new antibiotic, bypass the drug resistance, judicious use of the existing antibiotics, containment of drug resistance and through cutting edge CRISPR System. The recently developed and approved antibiotics like Dalvance (Dalbavancin), Sivextro (Tedizolid), Orbactiv (Ortavancin) are the outcome of high quality research in order to deal with the inevitable menace to the human kind in the form of AMR.
Key Words: Antimicrobial resistance (AMR), antibiotic, bacteria and drug resistance.
Introduction
Antibiotics have also helped to extend expected life span by changing the outcome of bacterial infections (Ventola 2015). Antimicrobial Resistance (AMR) occurs when bacteria, viruses, fungi and parasites no longer respond to medicines and increasing the risk of disease spread, severe illness and death. As a result of drug resistance, antibiotics and other antimicrobial medicines become ineffective and infections become increasingly difficult or impossible to treat (WHO). Antimicrobial substances selectively destroy or inhibit the growth of microorganisms. Further, it can be defined as molecules with diverse modes of action including the prevention of bacterial protein synthesis (Aminoglycosides, tetracyclines, macrolides and chloramphenicol, inhibition of nucleic acid synthesis (sulphonamides, quinolones and rifamycins), disruption of cell membrane function (polymyxins) and inhibition of bacterial cell wall synthesis (β-lactams and glycopeptides) (Larsson, and Flach,2022). The global use and misuse of antibiotics has led to the evolution and spread of bacterial resistance to all regularly used antibiotics.
The microorganisms are becoming extremely resistant to existing antibiotics and possibility of getting effective antibiotic is a distance dream as on today (Hart and Kariuki 1998). The global consumption of antibiotics has been increasing endlessly. The emergence of resistance to antimicrobial agent is becoming a major public health problem worldwide. Some of the major pathogens with antimicrobial resistance are Methicillin-Resistant Staphylococcus aureus (MRSA), Vancomycin-Resistant Enterococci, Drug-Resistant Streptococcus pneumoniae, Drug-resistant Mycobacterium tuberculosis, Carbapenem-Resistant Enterobacteriaceae, MDR Pseudomonas aeruginosa, MDR Acinetobacter, Extended-Spectrum Beta Lactamase (ESBL)-producing Enterobacteriaceae and Drug-resistant Neisseria gonorrhoeae (Ventola 2015b). In addition to their use in the treatment of infectious diseases, antibiotics are critical to the success of advanced surgical procedures, including organ and prosthetic transplants (Davies and Davies 2010).
Antimicrobial resistance (AMR) is a dangerous bacterial attribute that gives opportunities to survive and continue to grow instead of being inhibited or destroyed by therapeutic doses and hence results in severe consequences for public health. The incidence of drug resistance is increasing at an alarming rate and posing serious problems in the treatment of infectious diseases caused by multi-drug resistant (MDR) bacteria.
Key predisposing factors for antimicrobial resistance
The major predisposing factors, those are responsible for inevitable antimicrobial resistance include re-infection, poor drainage and perfusion as well as penetration of antimicrobial preparation. Apart from that, intracellular organism, malnutrition, hypoxia, acidosis, inappropriate route, substandard product, adverse effect, abuse of antimicrobial agents, nutritional imbalances, substandard nursing care, and improper predisposing management. Therefore, in this context, AMR will be the apparent outcome especially in India and the conditions cannot be ignored as long as our approach towards AMR control or prevention is not rational and universal.
Mechanism of antimicrobial resistance:
The bacteria generally attend the state of resistant to antimicrobial drugs through two ways such as natural/intrinsic and acquired. The natural way of antimicrobial resistance is being accomplished by the lack of metabolic process in the cell and also by nonappearance of binding or target site for antimicrobial drugs. But, the acquired antibiotic resistance is achieved broadly via two ways i.e. chromosomal mutation and biochemical mechanism. Plasmid mainly chromosomal mutation takes place within the bacteria or in between bacteria whereas resistances through biochemical mechanism include preventing enzyme and drug accumulation. Besides that, modifying target for antibiotic drugs, use of alternative pathways and quorum sensing are few under biochemical way for developing antimicrobial resistance (Fig.-1).
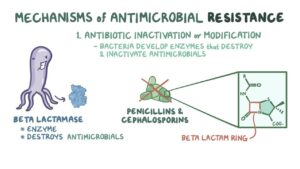
Fig.-1
Gene mediated antimicrobial resistance
Present studies on antimicrobial resistance of bacteria reveal that, the genes are mainly responsible for this type of morass in terms of antimicrobial resistance. The plasmid associated genes play the vital role for developing antimicrobial resistance. The intrinsic or natural resistance of an organism is a stable property encoded in the chromosome. The acquired resistance may develop either from changes or mutation in the genomic DNA or by acquisition of resistance genes through mobile genetic elements like plasmid, bacteriophage and transposons etc by gene transfer mechanisms such as conjugation, transformation, transduction and site specific integration.
Antimicrobial resistance genes emerge either by being mobilized from obscure strains or by evolving from obscure ancestral genes. Resistant genes may be situated on chromosomes, plasmids, integrons or on transposons . Varieties of mechanisms are being followed by the bacteria to evade the potent action of antibiotics. The bacteria destroy the antibacterial agent by acquiring genes encoding enzymes β-lactamases. It may acquire efflux pumps that extrude the antibacterial agent from the cell before it can reach its target site and exert its effect and by possessing several genes for a metabolic pathway, which ultimately produces altered bacterial cell walls that no longer contain the binding site of the antimicrobial agent. Besides that, bacteria may acquire mutations that limit access of antimicrobial agents to the intracellular target site via down regulation of porin genes. Thus, normally susceptible populations of bacteria may become resistant to antimicrobial agents through mutation and selection. The ability of bacteria to acquire and disseminate exogenous genes via mobile genetic elements has been the major factor in the development of MDR strains . Other than that, another mechanism for the dissemination of resistance involving integrons (Stokes and Hall, 1989) and are genetic elements that acquire and exchange exogenous DNA, known as gene cassettes, by a site-specific recombination mechanism . The increasing incidence of MDR micro-organisms has led to tremendous interest in the genetics and mechanisms of resistance evolved by bacteria to counteract the effects of antimicrobial agents. Reports from different countries have described a high prevalence of class 1 and class 2 integrons in Gram-negative clinical isolates which suggests that integrons are relatively common, especially in Enterobacteriaceae, and that they contribute to the spread of antimicrobial drug resistance in healthcare settings.
Novel and futuristic approach to tackle the AMR
- Develop new antibiotic
- Bypass the drug resistance
- Judicious use of the existing antibiotics
- Containment of drug resistance
- CRISPR System(Bacterial resistant gene editing)
Research and Development and use of new antibiotic: Use of recently developed antibiotics such as Dalvance (Dalbavancin), Sivextro (Tedizolid), Orbactiv (Ortavancin), Zerbaxa (Ceftolozane – Tazobactum) and Avycaz (Ceftazidime-avibactum) are providing promising results but abuse and under doses of these antibiotics may turn out to be a potential threats in terms of AMR.
By pass the drug resistance
Perhaps the most direct way to bypass resistance is to block the resistance mechanism. Resistance is frequently conferred by dedicated efflux pumps or antibiotic-degrading enzymes, which in turn can be countered by compounds that inhibit the resistance machinery. To use this strategy therapeutically, an antibiotic is delivered concurrently with resistance-inhibiting compounds; for example, a β-lactam antibiotic paired with an inhibitor of β-lactamase (a resistance enzyme that degrades β-lactams). This allows the antibiotic to kill both resistant and susceptible strains, thereby potentiating the efficacy of the drug and diminishing the selective advantage of the resistance gene. The most clinically successful examples are the pairings of amoxicillin-clavulanic acid, ampicillin-sulbactam, and pipericillin-tazobactam.
Judicious use of the existing antibiotics
The overuse of antibiotics is widely accepted as a major driver of some emerging infections. The growing emergence of multi-drug resistant organisms and the limited development of new agents available to counteract them have caused an impending crisis with alarming implications, especially with regards to Gram-negative bacteria including extended-spectrum beta-lactamase (ESBL)-producing E.coli and Klebsiella species,and carbapenem-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Inappropriate and injudicious use of antibiotics and other antimicrobials, as well as poor prevention and control of infections, are contributing to the development of AMR. It is absolutely necessary that every clinicians treating infections understands the underlying epidemiology and clinical consequences of AMR. The global nature of AMR calls for a global response, both in the geographic sense and across the whole range of sectors involved. Judicious, careful and rational use of antimicrobials is an integral part of good clinical practice. This attitude maximizes the utility and therapeutic efficacy of treatment, and minimizes the risks associated with emerging infections and the selection of resistant pathogens.
Containment of drug resistance
The WHO global strategy for containment of Antimicrobial resistance addresses this challenge through framework of interventions to slow the emergence and reduce the spread of antimicrobial-resistant microorganisms through: reducing the disease burden and the spread of infection and through improving access to appropriate antimicrobials. Besides that, it has taken steps towards strengthening health systems and their surveillance capabilities, enforcing regulations and legislation and encouraging the development of appropriate new drugs and vaccines. This strategy highlights aspects of the containment of resistance and it emphasizes on the need for further research directed towards filling the existing gaps in knowledge. Improving antimicrobial use must be a key action in efforts to contain resistance. Containment will require significant strengthening of the health systems in many countries and the costs of implementation will not be negligible. However, such costs must be weighed against future costs averted by the containment of widespread antimicrobial resistance.
CRISPR System (Bacterial resistant gene editing)
The CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) or Cas-9 System is considered as one of the ultimate products and it can be used as a lethal weapon in the war against antibiotic resistance in the present scenario.
- Produce programmable therapies of which targets antibiotic resistance genes directly,
- Use of bacteriophages to deliver a specific CRISPR Cas9 system into antibiotic-resistant bacteria and the antibiotic-resistant bacteria now sensitize the microbes to drugs.
- Modified version of phage therapy.
- CRISPR Cas-9 tool to deliver a specific DNA sequence to the targeted bacteria.
- Each spacer is typically derived from foreign genetic material (protospacer) and drives the specificity of CRISPR‐ mediated nucleic acid cleavage.
- In the target nucleic acid, each protospacer is associated with a protospacer adjacent motif (PAM) whose recognition is exclusive to individual CRISPR systems.
Advantages of CRISPR over traditional antibiotics
- CRISPR-induced double-stranded break (DSB) in the genome is lethal.
- If this DSB occurs on a plasmid, the plasmid will be eliminated from the bacterium, which may also induce cell death.
- It also enables increased specificity – rather than targeting a process essential to most bacteria,
- CRISPR antimicrobials can target specific sequences in a single virulent bacterial species, or even an antibiotic resistance gene.
- Native, non-pathogenic bacteria remain to help re-colonize the niche, reducing the chance of an opportunistic infection like for organisms like Clostridium difficile.
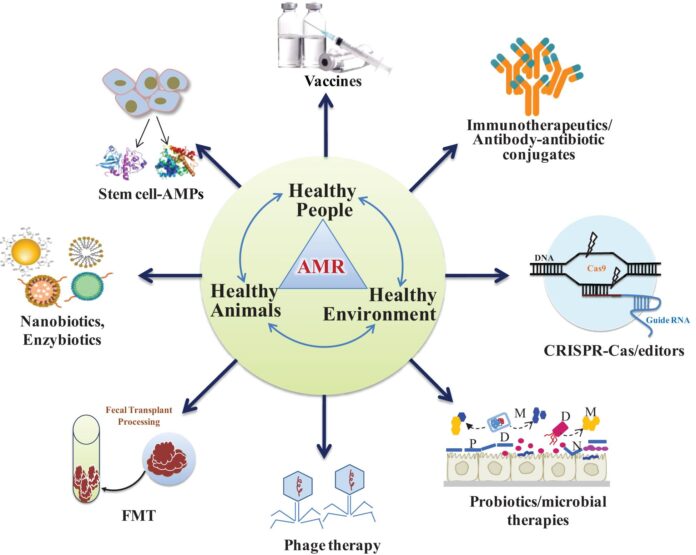
Advanced and futuristic approaches to be adopted as strategy for prevention of AMR
- Antibodies: antibodies, that binds and inactive pathogens, its virulence factors or its toxin. Apart from that, probiotics as live microorganisms that out compete harmful bacteria when administered in adequate amounts
- Lysins: Enzymes used by bacteriphage to destroy the cell wall of a target bacterium
- Wild type bacteriophages: Viruses that infect and kill bacteria.
- Engineered bacteriophages: Genetically engineered viruses with new properties to infect and kill bacteria.
- Immune stimulation: Innate proteins that have direct antibacterial activity.
- Vaccine: Inactivate bacteria or bacterial proteins that stimulate the immune system.
- Antimicrobial peptides: Small proteins that have direct antibacterial activity.
- Host defence and innate defence peptide: Peptides that increase expression of anti-inflammatory chemokines and cytokines, and reduce expression of pro-inflammatory cytokines
- Antibiofilm peptides: Peptides that specifically inhibit bacterial biofilm formation.
Conclusion
- Through billion years of evolution, microbes have developed myriad defense mechanisms designed to ensure their survival. This protection is readily transferred to their fellow life forms via transposable elements.
- Despite very early warnings, human have chosen to abuse the gift of antibiotics and have created a situation where all microorganisms are resistant to some antibiotics and some are resistant to all antibiotics.
- Finally antibiotics are “social drugs” that affect microbial resistance not only in the person taking the drug but also everyone else, because the resistance genes are easily passed to the community as well.
- Improving the hygiene in hospitals, screening of hospital visitors and isolating patients can control the spread of resistance for some extent.
- Improvements to clean water and sanitation, development of AMR surveillance capacity and optimization or minimization of antibiotic use in livestock farming could all stem the threat of AMR. These actions require funding, an understanding among policymakers of the significance of this threat and global collaboration.
References:
Davies J and Davies D (2010). Origin and evolution of antibiotic resistance. Microbiol and Mol Biol Revs 74 (3): 417-433.
Stokes H. W. and Hall R. M. (1989). A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Molecular Microbiology, (12): 1669-1683.
Hart CA and Kariuki S 1998. Antimicrobial resistance in Developing countries. British Medical Journal 317: 647-650.
Larsson, D.G.J., Flach, C F (2022). Antibiotic resistance in the environment. Nat. Rev. Microbiol. 20, 257–269.
Ventola CL (2015a). The antibiotic resistance crisis, Part I: Causes and threats. Pharmacy and Therapeutics 40 (4): 277-283.
Ventola CL (2015b). The antibiotic resistance crisis, Part II: Management strategies and new agents. Pharmacy and Therapeutics 40 (5): 344- 352.
*Corresponding Author: Dr. Debasish Behera,
Address: Assistant Professor, Department of Veterinary Pathology,
College of Veterinary Sciences and Animal Husbandry, R. K. Nagar, Tripura, West-799008, Email: behera.debasish1@gmail.com, Mo:7978901205