An overview of parasites of backyard poultry’s digestive tract in Meghalaya’s hill terrain
Meena Das1*, Rakesh Kumar2, Nampher Masharing and Mun Mun Makri
1ICAR Research Complex for NEH Region, Umiam, Meghalaya – 793103
2ICAR Research Complex for Eastern Region, Patna, Bihar – 800014
*Corresponding author: meenad3@gmail.com
Abstract
The current study set out to ascertain the frequency, species variety, and severity of gastrointestinal (G.I.) parasite infections in Meghalaya’s backyard poultry. A total of 1775 nos. of fecal samples were gathered from three age groups viz. < 8 weeks (523), those between 8-28 weeks (602), and > 28 weeks (650), and they were subjected to analysis using flotation, sedimentation, and modified McMaster methods. The genomic DNA corresponding to the 18S rRNA gene of Eimeria tenella was isolated, amplified, and sequenced. 37.97% was the overall percentage of G.I. parasite infections. Eight species of G.I. parasites were recorded i.e. Eimeria sp., Heterakis gallinarum, Ascaridia galli, Strongyloides avium, Capillaria sp., Raillietina sp., Syngamus and Choanotaenia infundibulum were recorded. Infection rates varied by age in birds that were less than eight (25.24%), between eight and twenty-eight (48.17%), and older than twenty-eight (387.76%) weeks. Eimeria sp. was found to be most prevalent in weeks < 8 (68.18%) and 8-28 (25.86%). The 18S rRNA small subunit gene of E. tenella was amplified using Polymerase Chain Reaction (PCR), which showed a band size of 1790 bp. After sequencing, the amplicon was submitted to the NCBI database. Sequence similarities between the E. tenella, Umiam, Meghalaya isolate with other sequences in the NCBI database were found to be greater than 99%, according to BLAST studies of the SSU rRNA gene. Nucleotide homology in pairwise alignment ranged from 71.59% to 100.0%. E. tenella isolates from Turkey and UK sharing the highest (100.0%) and lowest (95.6%) sequence homology, respectively.
Keywords: Parasites, Gastrointestinal tract, poultry, hilly, Meghalaya
Introduction
Animal rearing is an integral component of agriculture in India, and one of the most important parts for tribal farmers in Meghalaya is raising poultry in their backyards. The popularity of raising poultry in backyards is expanding quickly because of its cheap starting cost and readily available, affordable protein, and possibilities for employment (Frantovo, 2000; Bachaya et al., 2012). The 20th Livestock Census indicates that India’s poultry population was 851.81 million, representing a 16.8% growth compared to the previous census. According to the Livestock Census (2019), 317.07 million backyard chickens and 534.74 million commercial poultry are in the nation. Parasitism is a relationship in which the parasite relies on the host for metabolic processes, either completely or partially. Soulsby (1982) reported potential vectors for infection in poultry, including contaminated feed, water, litter, intermediary hosts, etc. The most common and damaging parasites that impact its production are those in the gastrointestinal tract or G.I. Bhowmik et al. (1982) found that parasitism leads to a loss in weight growth of up to 17% in developing chicks and a fall in egg production performance of 12.5%, in addition to causing significant morbidity and mortality in egg-laying hens. According to Jegede et al. (2015) and Afolabi et al. (2016) they are linked to catarrh, diarrhoea, intestinal blockage, decreased appetite, anaemia, weakness, insufficient feather growth in birds, as well as paralysis. The North Eastern region’s climate is ideal for the development and spread of several parasites. Das et al. (2024), Das et al. (2022), Das et al. (2021), and Das et al. (2020) reported the prevalence, diversity, and evolutionary connections of gastrointestinal parasites in backyard poultry from Meghalaya’s hilly region recently.
Materials and Methods
Study location and period: The investigation was carried out in the Meghalaya’s Ri Bhoi district, located between longitudes of 91°45´ and 92°15´ East and latitudes of 25°15´ and 26°15´ North. The area is distinguished for its rough and uneven terrain, encompassing a sequence of massifs. Four seasons were studied over the course of two years (2018-2019, 2019-2020): spring (March, April), monsoon (May, June, July, August, September), autumn (October, November), and winter (December, January, February). Identifying and classifying Eimeria tenella isolates using molecular techniques and analysing their evolutionary relationships was conducted in 2023-24.
Sample acquisition and analysis: Samples of fresh feces were gathered from multiple places in designated plastic pouches/vials. The birds were classified based on age into three categories: less than 8 weeks, 8 to 28 weeks, and above 28 weeks. 1775 nos. samples of poultry feces in total were obtained, categorised by age groups: less than 8 weeks (523 samples), 8 to 28 weeks (602 samples), and above 28 weeks (650 samples). The direct flotation method was employed to analyze the fecal samples, utilizing saturated salt and sucrose solution (Soulsby, 1982). The modified McMaster technique (MAFF, 1986) was then applied to the positive samples in order to calculate the number of eggs per gram (EPG) and oocysts per gram (OPG) of feces. Samples that were not analyzed on that particular day were kept in a refrigerator at 4°C so that they may be analyzed the next day. Oocyst sporulation was accomplished by combining positive samples containing Eimeria oocysts with a 2.5% potassium dichromate solution (Sloss et al., 1994). Microscope was used to investigate the morphology of the oocysts with magnifications 10x, 20x and 40x. An autopsy was performed on the deceased chicks. Microscopic examination of coccidia oocysts was performed using wet smears prepared from deep scrapings taken from the different parts of small and large intestine in accordance with the protocol outlined by Flek and Moody (1993).
Isolation of genomic DNA: Prior to DNA extraction, the sporulated oocysts of E. tenella were subjected to several rounds of centrifugation and resuspension in distilled water to eliminate potassium dichromate. The oocysts are subjected to 3-4 rounds of centrifugation at a speed of 13000rpm for 5 minutes each, using distilled water for cleaning. The pellet containing 5000 oocysts was then subjected to the glass bead DNA extraction methodology (Reginato et al., 2020) with a little modification, which included repeatedly thawing (at 57°C) and freezing (at -80°C) until the oocyst wall was broken. The genomic DNA was extracted and dissolved in 100µl of 1X TE buffer. Thermo-Fisher Scientific’s NanoDrop 1000 UV-Vis Spectrophotometer was used to measure the concentration of genomic DNA. Additional verification was conducted using gel electrophoresis and ethidium bromide-stained agarose gel (0.8%).
Amplification and sequencing: Primers unique to a particular species were utilized to amplify the 18S rRNA SSU of the extracted DNA, as previously reported by Schwarz et al. (2009). A 25μL reaction mixture, including 12.5μL of the master mix (2X), 2μL of the genomic DNA template, 1μL of each primer, and 8.5μL of nuclease-free water, was used for the amplification.
A thermal cycler was used to carry out the amplification procedure in accordance with a cyclic protocol. A 5 minutes initial denaturation stage at 95°C and a 60 seconds subsequent denaturation step at the same temperature made up the procedure. After that, an annealing phase was carried out for 60 seconds at 62°C. An extension step at 72°C for 120 seconds came next.
The last extension step was carried out for 5 minutes at 72°C. The PCR product was exposed to a UV transilluminator, electrophoresed on a 1% agarose gel, and stained with ethidium bromide. Using a 1 kilobase DNA ladder, the size of the PCR product was confirmed. The Sanger sequencing technique was used to sequence the PCR-generated fragments.
Phylogenetic analysis: The Basic Local Alignment Search Tool (BLAST) was utilized to confirm the nucleotide sequence of E. tenella, which was ascertained through sequencing (http://blast.ncbi.nlm.nih.gov/). The CLUSTAL W algorithm (Thompson et al., 1994) from the Molecular Evolutionary Genetic Analysis (MEGA X) computer package was then used to align the sequence. The evolutionary history of the analysed taxa was determined using the Maximum Parsimony and Neighbor-Joining Methods (Saitou and Nei, 1987), respectively. A bootstrap value of 1000 repetitions (Felsenstein, 1985) was used to illustrate the evolutionary history.
Results and Discussion
The backyard poultry in the hilly area of Meghalaya had a prevalence rate of 37.97% G.I. parasitic infection, as shown in Table 1 (Das et al., 2022). The study documented eight species of gastrointestinal parasites, namely Eimeria sp. (30.12%), Heterakis gallinarum (14.09%), Ascaridia galli (21.22%), Capillaria sp. (7.57%), Syngamus trachea (3.56%), Raillietina sp. (8.61%), Choanotaenia infundibulum (2.37%), and Strongyloides avium (12.46%) (Fig.1).
Table 1: Prevalence of G.I. parasites in backyard poultry
| Age
(weeks) |
Sample examined | Sample positive | Eimeria sp. | H. gallinarum | A. galli | Capillaria sp. | S. trachea | Raillietina sp. | Choanotaenia sp. | S. avium |
| <8 | 523 | 132
(25.24) |
90
(68.18) |
24
(18.18) |
– | 18
(13.64) |
– | – | – | – |
| 8-28 | 602 | 290
(48.17) |
75
(25.86) |
41
(14.13) |
74
(25.52) |
20
(6.89) |
10
(3.45) |
20
(6.89) |
10
(3.45) |
40
(13.79) |
| > 28 | 650 | 252
(38.77) |
38
(15.08) |
30
(11.90) |
69
(27.38) |
13
(5.16) |
14
(5.56) |
38
(15.08) |
6
(2.38) |
44
(17.46) |
| Total | 1775 | 674
(37.97) |
203
(30.12) |
95
(14.09) |
143
(21.22) |
51
(7.57) |
24
(3.56) |
58
(8.61) |
16
(2.37) |
84
(12.46) |
Figures in parentheses indicates percent positivity
 |
 |
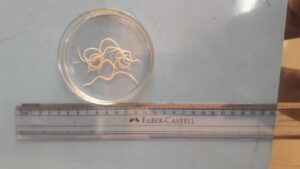 |
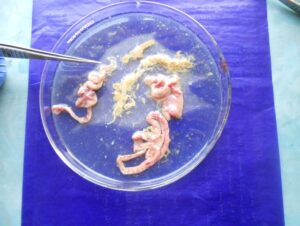
 |
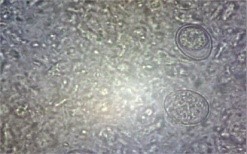
| Tapeworms in small intestine | Tape worms
(Raillietina sp.)
|
Round worms
(Ascaridia galli)
|
Caecal worm
(Heterakis gallinarum) |
 |
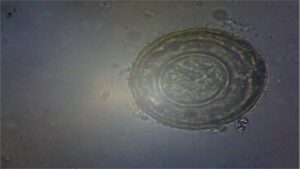 |
 |
 |
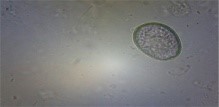
| Threadworm Egg
(Capillaria sp.) |
Tape worms Egg
|
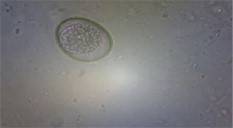
Ascaridia galli egg
|
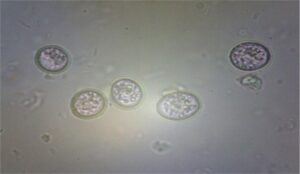
Eimeria oocyst |
Fig. 1 G.I. parasite eggs and oocysts in backyard poultry
According to the current study, G.I. parasitic infections have been reported in Chhattisgarh, Odisha, Jammu, and Madhya Pradesh by Kumari et al. (2018), Hembram et al. (2015), Katoch et al. (2012), and Naphade and Chaudhari (2013), respectively, with prevalence rates of 25%, 58.75%, 72%, and 84.05%. The variance in the percentage prevalence observed in this study could be ascribed to variations in the farmers’ farming practices, environmental factors, and geographic location. The greatest infection rate was reported in October (52.88%), while the lowest was recorded in February (26.34%). The number of eggs per gram (EPG) in feces, which is a measure of infection intensity, ranges from 50 to 550. Many factors, including the environment, management practices, level of biosecurity, and the availability of intermediate hosts and reservoirs, all have an impact on the high prevalence of parasitic ailments in poultry (Catelli et al., 1999; Permin et al., 1999). According to Taylor et al. (2016), 26-29°C is the optimal temperature range for the growth and hatching of eggs or oocysts, and a relative humidity of more than 80% is also necessary.
Parasitic infections in poultry were seen across all age categories, including those less than 8 weeks (25.24%), between 8 and 28 weeks (48.17%), and more than 28 weeks (38.77%) (Table 1). During the 8-28 week period, all eight species of gastrointestinal parasites were found in the birds. However, the percentage of infection in birds older than 28 weeks is lower compared to birds aged between 8 and 28 weeks. Specifically, the infection rates for A. galli, S. avium, Eimeria sp., Raillietina sp., H. gallinarum, S. trachea, Capillaria sp., and C. infundibulum are 27.38%, 17.46%, 15.08%, 15.08%, 11.90%, 5.56%, 5.16%, and 2.38%, respectively. Age-based differences in the prevalence of gastrointestinal parasite infections in poultry have also been reported by Islam et al. (2020) and Wokem and Obiyor (2018). Young birds’ increased disease incidence could be linked to weakened immune systems as well as ongoing exposure to pathogens via polluted litter.
The total prevalence of Eimeria sp. was 30.12% (Table 1). Das et al. (2021) reported eight species of Eimeria viz. E. tenella, E. necatrix, E. maxima, E. mitis, E. brunetti, E. praecox, E. mivati and E. acervulina by morphological characterization from hilly region of Meghalaya (Fig. 2). August (30000) and February (9500) had the greatest and lowest recorded oocyst per gram (OPG), respectively. According to Long et al. (1976), E. tenella, E. brunetti and E. necatrix are linked to hemorrhagic coccidiosis and have a high death and morbidity rate. They can also be extremely pathogenic. Although morbidity and mortality can occur depending on the dose consumed, parasite strain-specific variation in virulence, and host factors like age, breed, and immune status, E. acervulina, E. maxima, E. mitis, and E. praecox are less pathogenic and cause malabsorptive pathologies (Williams et al., 2009). Williams et al. (1996) state that co-infection with multiple species is frequent and can make diagnosis more difficult. Although E. tenella is more prevalent and has a bigger effect on poultry productivity, E. necatrix has been identified as the most harmful Eimeria sp. (Blake et al., 2015).
 |
 |
 |
|
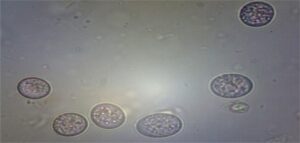
|
E. mitis |
E. brunetti | E. praecox | E. tenella |
1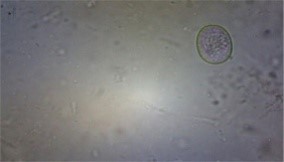 |
 |
 |
 |
| E. mivati | E. necatrix | E. maxima | E. acervulina |
Fig.2 Various types of Eimeria species in backyard poultry of Meghalaya
Omer et al. (2011) state that while coccidiosis can affect birds of any age, it tends to affect younger birds more than older ones. This may be due to the immature immune systems of young birds, which make them vulnerable to infection even from less pathogenic or lower grade strains of Eimeria species. Morris and Gasser (2006) state that Eimeria sp. proliferate in the intestinal system, resulting in tissue damage, disruption of feeding and digestive functions, loss of blood, and heightened vulnerability to other pathogens. Clinical coccidiosis outbreaks are caused by a number of factors, such as litter moisture levels above 30%, immunological suppression, inadequate anticoccidial inclusion in feed, and stress from the environment and management (Singla et al., 2007).
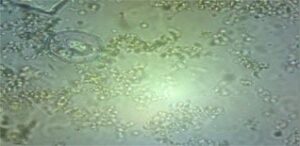
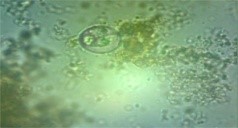
- tenella is the cause of caecal coccidiosis, which is characterized by severe caecal hemorrhages and bloody diarrhea (Fig. 3).
|
|
|

Mortality in chicks (3-4 weeks) |

PM examination of chicks |
|
|
|

Distension of caeca due caecal coccidiosis |
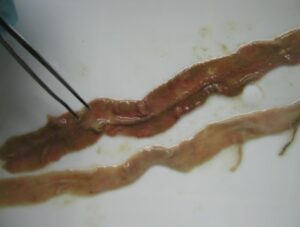
Haemorrahage and sloughing of caecal mucosa |
Fig. 3 Caecal coccidiosis in chicks (3-4 weeks)
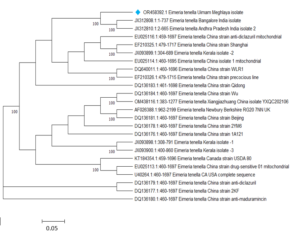
The 18S rRNA small subunit gene of E. tenella was amplified using Polymerase Chain Reaction (PCR) as stated by Schwarz et al. (2009), which showed a band size of 1790 bp. After sequencing, the amplicon was submitted to the NCBI database. Sequence similarities between the E. tenella, Umiam, Meghalaya isolate with other sequences in the NCBI database were found to be greater than 99%, according to BLAST studies of the SSU rRNA gene. Nucleotide homology in pairwise alignment ranged from 71.59% to 100.0%. E. tenella isolates from Turkey and UK sharing the highest (100.0%) and lowest (95.6%) sequence homology, respectively. Thenmozhi et al. (2013) also found a comparable level of similarity in the nucleotide sequences of E. tenella’s ITS-1 region. The sequences were obtained from faeces collected from Tamil Nadu, India farms. The American and European strains showed nucleotide homology ranging from 95% to 100%. The current investigation detected a similar sequence homology of SSU rRNA across various E. tenella isolates from Kerala, similarly reported by Thenmozhi et al. (2014).
Fig. 4 Phylogenetic tree based on E. tenella isolates’ 18S rRNA gene
Conclusion
The current investigation revealed that backyard poultry in the subtropical hills of Meghalaya were consistently infected with a wide variety of gastrointestinal parasites. Eimeria has eight predominant species, and infections are most often observed during the monsoon season. The infection has a significant likelihood of affecting younger age groups. Regular screening and administration of anthelmintic and anticoccidial medications are essential for ensuring profitability in backyard poultry production.
Acknowledgements
The Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi, is acknowledged by the authors for its financial support. They would also like to thank the Director, ICAR Research Complex for NEH Region, Umiam, Meghalaya, for overseeing the research work carried out under projects (OXX5395) and (IXX15052).
References
Afolabi O.J., Simon-Oke I.A. and Olasunkanmi A.O.; 2016. Intestinal parasites of domestic
chicken (Gallus gallusdomesticus) in Akure, Nigeria. J. Biomed., 1(4): 1-4.
Bachaya H.A., Raza M.A., Khan M.N., Iqbal Z., Abbas R.Z., Murtaza S. and Badar N.; 2012.
Predominance and detection of different Eimeria species causing coccidiosis in layer chickens. J. Anim. Pl. Sci., 22: 597-600.
Bhowmik M.K., Sasmal N.K. and Chakraborty A.K.;1982. Effect of Raillietina cesticellus
infection on the meat and egg production of fowl. Indian Vet. Med. J., 6(2): 100-102.
Blake D.P., Clark E.L., Macdonald S.E., Thenmozhi V., Kundu K., Garg R., Jatau I.D., Ayoade
S., Kawahara F., Moftah A., Reid A.J., Adebambo A.O., Zapata R.A., Srinivasa Rao A.S.R., Thangaraj K., Banerjee P.S., Raj G.D., Raman M. and Tomley F.M.; 2015. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. PNAS, https://doi.org/10.1073/pnas.1506468112.
Catelli C.T.E., Poglayen G. and Gadale A.T.O.; 1999. Preliminary study of the helminthes of
The chicken digestive tract in Somalia. Pathologie Infect., 52: 107-112.
Das M., Masharing N. and Makri M.M.; 2024. Phylogenetic analysis of Eimeria tenella isolates
from chicken of sub-tropical mountains of Meghalaya, India. Mol. Biol. Rep., 51: 110. https://doi.org/10.1007/s11033-023-09181-y.
Das M., Laha R. and Doley S.; 2022. Gastrointestinal parasites in the backyard poultry of mid-
hill region of Meghalaya. Indian J. Anim. Sci., 92 (2): 179-182.
Das M.; 2021. Diversity of Eimeria species in backyard poultry of subtropical hilly region of
Meghalaya, India. J. Entomol. Zool. Stud., 9(2): 360-365.
Das M., Laha R. and Doley S.; 2020. Gastrointestinal parasites in backyard poultry of
subtropical hilly region of Meghalaya. J. Entomol. Zool. Stud., 1301-1305: 8(5).
Fleck S.L. and Moody A.H.;1993. Diagnostic technique in medical parasitology, 11th Edition.
Cambridge: Cambridge University Press. pp. 10-14.
Felsenstein J.; 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evol.,
39(4): 783-791.
Frantovo D.; 2000. Some parasitic nematodes (Nematoda) of birds (Aves) in the Czech
Republic. Acta Soc. Zool. Bohem., 66(1): 13-28.
Hembram A., Panda M.R., Mohanty B.N., Pradhan C.R., Dehuri M., Sahu A. and Behera M.;
- Prevalence of gastrointestinal helminths in Banaraja fowls reared in semi-intensive system of management in Mayurbhanj district of Odisha. Vet. Wrld., 8(6): 723-726.
Islam M.S., Dey A.R., Parvin S., Farjana T. and Alam M.Z., 2020. Intestinal parasitic infection
in commercial chickens in Sirajgonj. J. Bang. Agri. Univ., 18(1): 111-116.
Jegede O.C., Asadu I.A., Opara M., Obeta S.S. and Olayemi D.O.; 2015. Gastrointestinal
parasitism in local and exotic breeds of chickens reared in Gwagwalada Guinea Savannah zone of Nigeria. Sokoto J. Vet. Sci., 13(3): 25-30.
Kumari B., Pal S., Sanyal P.K. and Verma S.K.; 2018. Studies on Prevalence of Gastrointestinal
Helminthic Infections in Poultry of Durg (Chhattisgarh). Intern. J. P. Appl. Biosci., 6(3): 570-574.
Katoch R., Yadav A., Godara R., Khajuria J.K., Borkataki S. and Sodhi S.S.; 2012. Prevalence
and impact of gastrointestinal helminths on body weight gain in backyard chickens in subtropical and humid zone of Jammu, India. J. Parasit. Dis., 36: 49-52.
Long P., Joyner L., Millard B. and Norton C.; 1976. A guide to laboratory techniques used in the
study and diagnosis of avian coccidiosis. Folia Vet. Latin., 6: 201-217.
MAFF; 1986. Ministry of Agriculture, Fisheries and Food. Manual of veterinary parasitological
techniques, Her Majesty’s Stationery Office, London.
Morris G.M. and Gasser R.B.; 2006. Biotechnological advances in the diagnosis of avian
coccidiosis and the analysis of genetic variation in Eimeria. Biotech. Adv., 24(6): 590-603.
Naphade S.T. and Chaudhari K.V.; 2013. Studies on the Seasonal Prevalence of Parasitic
Helminths in Gavran (Desi) Chickens from Marathwada Region of Maharashtra. Intern. J. Faun. Biol. Stud., 1(2): 4-7.
Omer S.A., Apio A., Wronski T. and Mohammad O.B.; 2011. A new coccidian parasite (Eimeria
farasanii n. sp.) indicates parasite-host specificity in endemic Farasan gazelle. Intern. J. Zool. Res., 7: 85-92.
Livestock Census; 2019. 20th Livestock Census. Department of Animal Husbandry, Dairying
and Fisheries, Ministry of Agriculture, Government of India.
Permin A., Bisgaard M., Frandsen F., Pearma M., Kold J. and Nansen P.; 1999. Prevalence of
gastrointestinalhelminths in different poultry production systems. British Poul. Sci., 40 (4): 439 – 443.
Singla L.D., Pangasa A. and Juyal P.D.; 2007. Caecalcoccidiosis: efficacy of ayurvedic and
allopathic coccidiostats in immunomodulated broiler chicks. Proceedings of the 12th International Conference of the Association of Institutions of Tropical Veterinary Medicine held from August 19-22, 2007 at Montpellier, France.
Sloss M.W., Kemp R.L. and Zajac A.M.; 1994. Veterinary Clinical Parasitology. 6th Edition.
Iowa State University Press, Ames, Iowa.
Soulsby E.J.; 1982. Helminths, Arthropods and Protozoan’s of Domesticated Animals. 7th ed.
BailliereTindall, London.
Schwarz R.S., Jenkins M.C., Klopp S. and Miska K.B.; 2009. Genomic Analysis of Eimeria spp.
Populations in Relation to Performance Levels of Broiler Chicken Farms in Arkansas and North Carolina. J. Parasitol., 95: 871-880.
Saitou N. and Nei M.; 1987. Theneighbor-joining method: a new method for reconstruction of phylogenetic trees. Mol. Bio. Evo., 4: 406-25.
Reginato C.Z., Braunig P., Portella L.P., Mortari A.P.G., Minuzzi C.E., Sangioni L.A., Vogel F.
S.F.; 2020. DNA extraction methods for molecular detection of Eimeria spp. in cattle and sheep. Pesqui. Vet. Brasil., 40(7): 514-518.
Taylor M., Coop R. and Wall R.; 2016. Veterinary Parasitology. 4thEdn: Wiley Blackwell.
Thenmozhi V., Sureshkumar M., Raman M., Gomathinayagam S. and Veerakumari L.; 2013.
Molecular characterization of Coimbatore isolates of Eimeria tenella by ITS-1 based nested PCR. Indian Vet. J., 90 (11): 22-23.
Thenmozhi V., Veerakumari L. and Raman M.; 2014. Preliminary Genetic Diversity Study on
Different Isolates of Eimeria tenella from South India. Intern. J. Adv. Vet. Sci. Tech., 3(1): 114-118.
Wokem G.N. and Obiyor E.T.; 2018. Assessment of Intestinal Parasites of Commercial Layers in
Selected Local Government Areas of Rivers State, Nigeria and Their Public Health Implications. Cur. Trd. Biomed. Engin. Biosci., 11(5): CTBEB.MS.ID.555822.
Williams R.B., Marshall R.N., Pages M., Dardi M. and Cacho E.; 2009. Pathogenesis of Eimeria
praecox in chickens: virulence of field strains compared with laboratory strains of E. praecox and E. acervulina. Avian Pathol., 38: 359-366.
Williams R.B., Bushell A.C., Reperant J.M., Doy T.G., Morgan J.H., Shirley M.W., Yvore P.,
Carr M.M. and Fremont Y.; 1996. A survey of Eimeria species in commercially-reared chickens in France during 1994. Avian Pathol., 25:113-130.