Aetiology and Global Prevalence of Lumpy Skin Disease
K.L. Dahiya
Veterinary Surgeon, Department of Animal Husbandry & Dairying, Haryana
Abstract
Lumpy skin disease, characterized by the onset of high fever and nodule formation over the skin and huge losses to livestock farmers, first time reported in Zambia of Africa in 1929 recognized as pseudo-urticaria, is caused by Lumpy skin disease virus of capripox virus genus, poxviridae family, subfamily Chordopoxvirinae. Since it’s reported from its original foci in Zambia spread to entire African continent the disease was also reported in 1984 from middle-east, in 2013 south‐eastern Europe and in 2019 East Asian countries. Now, the virus is prevalent in most of the parts of world.
Keywords: Lumpy skin disease, Cattle, Virus, Prevalent, Africa, Asia, Europe.
Introduction
Lumpy skin disease (LSD) is an infectious disease caused by Lumpy skin disease virus (LSDV) affecting bovines particularly the cattle in the world. It is a vector-borne disease transmitted by different biting and blood feeding arthropods. Until 1989, LSD is limited to African continent. However, the disease is moved outside Africa to Madagascar and the other parts of the world. LSD causes considerable economic losses due to emaciation, damage to hides, infertility, mastitis, loss of milk production, and mortality. The incubation period ranges from one to four weeks (Das et al. 2021). Fever is the initial sign that is followed within 1-2 days by the development of nodules on the skin and mucous membranes. A diagnosis of LSD is building upon the basis of the typical clinical patterns (morbidity and mortality). Supportive treatment should be given to infected animals to relieve clinical signs and to control all secondary complications. Immunization of the susceptible animals is the effective methods to control the disease.
The original foci of LSD are from Zambia in 1929. LSD is considered as an endemic disease in the African continent. After 1984, the disease has been moved beyond Africa. It is reported in Madagascar and some countries in the Arab Gulf Peninsula and Middle East and Europe (Russia) and East Asian countries.
Synonyms of LSD
Lumpy skin disease in abbreviated form is known as LSD. The other synonyms of the disease are Pseudo-urticaria, Lumpy Disease, Ngamiland cattle disease, Neethling virus disease, exanthema nodularis bovis, and knopvelsiekte.
Aetiology and Classification
The Lumpy skin disease virus (LSDV) is aetiological agent of Lumpy skin disease (LSD). LSDV belongs to genus Capripoxvirus of the family Poxviridae (subfamily Chordopoxvirus). Although, LSDV is closely related antigenically to sheep pox and goat poxviruses but these three viruses are distinct, they cannot be easily differentiated with routine serological tests. According to the international committee on Taxonomy of LSD virus is summarized in table 1:
| Table 1. Virus classification | |
| (unranked) | Virus |
| Realm | Varidnaviria |
| Kingdom | Bamfordvirae |
| Phylum | Nucleocytoviricota |
| Class | Pokkesviricetes |
| Order | Chitovirales |
| Group | Group I (Double stranded DNA) |
| Family | Poxviridae |
| Subfamily | Chordopoxvirinae |
| Genus | 1. Avipoxvirus
2. Capripoxvirus 3. Centapoxvirus 4. Cervidpoxvirus 5. Crocodylidpoxvirus 6. Leporipoxvirus 7. Macropopoxvirus 8. Molluscipoxvirus 9. Orthopoxvirus 10. Oryzopoxvirus 11. Parapoxvirus 12. Pteropopoxvirus 13. Salmonpoxvirus 14. Sciuripoxvirus 15. Suipoxvirus 16. Vespertilionpoxvirus 17. Yatapoxvirus |
| Species (of genus Capripoxvirus): | 1. Sheeppox virus
2. Goatpox virus 3. Lumpy skin disease virus |
| Source(s): Schoch et al. 2020; Hulo et al. 2011 | |
Capripoxviruses are not easily distinguishable morphologically from Orthopoxviruses and serologically, these viruses share antigens with parapoxviruses which are attributed due to the genetic similarity between the viruses (Roy et al. 2008)
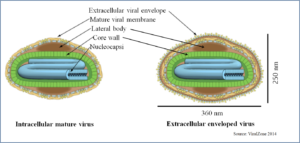
Structure of LSDV
Including LSDV Capripoxviruses in the Poxviridae family are large brick shaped, enveloped with complex symmetry 300 x 270 x 200 nm in size. The surface membrane displays surface tubules or surface filaments. The virus has two distinct infectious virus particles i.e. the intracellular mature virus and the extracellular enveloped virus (ViralZone 2014, Schoch et al. 2020). The large, double-stranded DNA (dsDNA) virus is very stable, and very little genetic variability occurs. Therefore, for LSDV, farm-to-farm spread cannot be followed by sequencing the virus isolates, as is done with other transboundary diseases, e.g. foot-and-Mouth disease (Tuppurainen et al. 2017).
Difference between Chordopox viruses
Including chordopox viruses, all the pox viruses are enveloped, double stranded (ds), brick or ovoid shaped, 220-450 nm long and 140-260 nm wide, with 130-375 kb genome size (ViralZone 2014).
| Subfamily | Genra | |
| Taxonomic hierarchy | Chordopoxvirinae | Capripoxviruses |
| Envelop | Present | Present |
| Shape | Brick-shaped or ovoid virion | Brick-shaped |
| Size | 220-450 nm long and 140-260 nm wide | 300 x 270 x 200 nm |
| Genome | Linear, dsDNA genome of 130-375 kb | Linear, dsDNA genome of about 154 kb |
Genome of LSDV
- The virion consists of more than 90% protein, 3.2% double stranded DNA (Fenner et al. 1974).
- The LSDV genome (151-kbp – Kilobase pairs) consists of a central coding region bounded by identical 2.4 kbp-inverted terminal repeats and contains 156 putative genes (Tulman et al. 2001).
- The LSDV genes share a high degree of colinearity and amino acid identity (average of 65%) of its genomic region with genes of other known mammalian poxviruses, particularly suipoxvirus, yatapoxvirus, and leporipoxviruses (Tulman et al. 2001).
- Although LSDV resembles leporipoxviruses in gene content and organization, it also contains homologues of interleukin-10, interleukin-1 binding proteins, G protein-coupled CC chemokine receptor, and epidermal growth factor-like protein which are found in other poxvirus genera. LSDV is closely related to other members of the Chordopoxvirinae, it contains a unique complement of genes responsible for viral host range and virulence (Tulman et al. 2001).
Susceptible of LSDV
The LSDV is susceptible to different levels of temperature, pH, chemicals and environmental conditions (OIE 2017).
- Temperature: LSDV is susceptible to 55°C for 2 hours and 65°C for 30 minutes. It can be recovered from skin nodules and kept at –80 °C for 10 years. The infected tissue culture fluid can be stored at 4°C for 6 months.
- pH: The virus is susceptible to highly alkaline or acid pH. However, there is no significant reduction in titre when held at pH 6.6–8.6 for 5 days at 37°C.
- Chemicals: LSDV is susceptible to 20% ether, chloroform, 1% formalin, and some detergents, e.g. sodium dodecyl sulphate. It is also susceptible to 2% phenol for 15 minutes, 2-3% sodium hypochlorite, 1:33 dilution of iodine compounds and 0.5% quarternary ammonium compounds.
Survivability of LSDV
LSDV has remarkably stable, surviving for long periods at ambient temperature, especially in dried scabs. It is very resistant to inactivation. It is surviving in necrotic skin nodules for up to 33 days or longer, desiccated crusts for up to 35 days and at least 18 days in air-dried hides. It can remain viable for long periods in the environment. Meanwhile, the virus is susceptible to sunlight and detergents containing lipid solvents, while, in dark environmental conditions, such as contaminated animal sheds, it can persist for many months (OIE 2017).
History and Geographic Distribution of LSDV
1929: Long before the discovery of aetiological agent of LSD was known, the first description of the clinical signs of this disease was explained in 1929 in Zambia (formerly Northern Rhodesia) in cattle described as “pseudo-urticaria”. At that time the disease was thought to be caused by the bites of insects (MacDonald 1931).
1943: An outbreak of this disease occurred in Ngamiland during 1943 as Ngamiland cattle disease (Von Backstrom 1945).
1943 & 1945: Same clinical signs were occurred in Botswana, Zimbabwe and the Republic of South Africa between 1943 and 1945, where the infectious nature of the disease was recognized in these outbreaks.
1944: Towards the end of 1944 the disease made its first appearance in the Transvaal and then spread rapidly throughout South Africa during the next years, despite enforced control measures (Weiss 1968).
1945: The disease continued to appear in later year of 1945 and was also attributed to plant poisoning (Le Roux 1945).
1945: This disease was first recognized as an infectious malady by Von Baokstrom (1945), when an outbreak occurred in Ngamiland during 1943.
1945: Thomas, Robinson and Alexander (1945) were the first to demonstrate the transmissibility of the infectious agent by the subinoculation of suspensions of skin nodules (Thomas et al. 1945, Weiss 1968).
1947: By 1947 the disease had become firmly established and enzootic in South Africa and had also been reported from Swaziland, Basutoland and Portuguese East Africa and subsequently from Madagascar, Tanganyika and the Belgian Congo (Diesel 1949).
1948: Van Den Ende, Alexander, Don and Kipps isolated a virus in embryonated eggs, which they believed to be the cause of LSD, but which was subsequently shown to have no etiological relationship to the disease (Alexander et al. 1957, Haig 1957, Weiss 1968).
1957: The first outbreak of the disease in Kenya occurred towards the end of 1957 and affected indigenous Zebu cattle as well as exotic breeds (MacOwen 1959).
1957: The virus causing LSD was first isolated in tissue culture by Alexander et al. (1957). Subsequently it was recovered on numerous occasions from the skin lesions of infected cattle in South Africa as well as in Kenya and there is no doubt that the Group III viruses (prototype Neethling), described by Alexander et al. 1957, is the cause of LSD (Prydie & Coackley 1959).
1971: The disease was observed in August, 1971 in Western Sudan in epizootic proportions and spread to the Eastern Sudan and in 1972 continued in the country (Ali & Obeid 1977).
1973: The disease was recognized in Chad (Nawathe et al. 1978).
1974: The disease was reported in West Africa including Nigeria (Nawathe et al. 1978)
1983: The disease was spreading into Somalia in 1983 (Davies 1991).
1981–1986: Between 1981 and 1986 outbreaks of epizootic LSD were reported in Tanzania, Kenya, Zimbabwe, Somalia and the Cameroon with mortality rates of 20% in affected cattle (Brenner et al. 2006). The disease was restricted to some countries in sub-Saharan Africa between 1929 to 1986 (Brenner et al. 2006).
1984: From 1984 to 1988, there were unconfirmed reports of the disease in cattle in Oman and Kuwait (Kasem et al. 2018).
1986: The first report outside Africa was described in Kuwait in 1986, where 642 cases were reported in cattle (Brenner et al. 2006). The disease was again reported from Kuwait in 1991 (Kumar 2011).
1988: The first confirmed LSDV cases within Middle East countries were reported in Egypt where it subsequently became endemic (Kasem et al. 2018).
1989: A LSD outbreak also occurred in August and September 1989 in Israel at village Peduyim and two neighbouring villages. Circumstantial evidence suggested that the infection was transmitted by stable flies (Stomoxys calcitrans) carried by the wind from foci of the disease at Arish in northern Sinai or Ismailiya and the Nile delta in Egypt (Yeruham et al. 1995). The outbreak was controlled by culling all the animals in the village and performing ring vaccination with a sheep pox vaccine (Yeruham et al. 1995, Brenner et al. 1992). Additionally, Israeli investigators assessed the efficacy of the sheeppox vaccine, RM-65 strain, by performing a controlled challenge study with vaccinated and non-vaccinated calves in the high containment unit of the Kimron Veterinary Institute (Brenner et al. 1992). The Israeli wild-type strain that was first isolated by Abraham and Zissman in 1989 served as the challenge virus (Abraham & Zissman 1991).
1989: In mid-August 1989, the first case of a LSD infection reported in the Arabian onyx (Oryx leucoryx) from Saudi Arabia (Greth et al. 1992). The outbreak of LSD was also reported from Israel (Kumar 2011).
1993: The cases of LSD were reported from Bahrain in 1993 but were not confirmed by virus isolation (Kitching 2004).
2000: Yemen, United Arab Emirates in 2000 and the West Bank also reported LSD invasion (Shimshony & Economides 2006; Kumar 2011; Wainwright et al. 2013).
2001: In 2001, LSD was reported in Mauritius, Mozambique and Senegal.
2002-03: The LSD outbreak again reported on Bahrain (Kumar 2011).
2006: LSDV was reintroduced into Egypt and Israel (Kasem et al. 2018). The cases of LSD were reported from Egypt during the months of April, May and June 2006 (Salib & Osman 2011).
2007–11: Active outbreaks of LSD in African continent were occurred in central Ethiopia in 2007 to 2011 in an investigation done in four districts i.e. Adama, Wenji, Mojo and Welenchiti. The totally 1675 outbreaks were reported over 5 years period from 2007 to 2011, with 62176 cases and 4372 deaths. The Oromia represented the highest numbers of outbreaks (1066), followed by Amhara (365) and the Southern Nations, Nationalities and People’s Region (123). During the year 2010 the highest number of outbreaks were frequently seen between September and December. The morbidity and mortality rates were 13.61% (296) and 4.97 % respectively (Ayelet et al 2014).
2009: The confirmed cases of the LSD were reported in 2009 from Oman in a farm population of 3200 Holstein animals with a high morbidity and mortality rates 30-45% and 12% respectively (Kumar 2011, Tageldin et al. 2014).
2011–12: Abattoir records in Egypt showed a wide spectrum data concerning LSD occurring in traditional livestock herds (Ahmed & Dessouki 2013).
2012-13: Between August and October 2013, four LSD outbreaks were reported in Turkey along the eastern boundary of the southern border with the Syria, Iraq and Iran. This outbreak followed the LSD outbreaks in Israel, Lebanon, the West Bank and Jordan, which were reported between Mid April 2012 and September 2013 (Wainwright et al. 2013). In Jordan, the overall morbidity rate was 26%, mortality rate 1.9% and case fatality rate 7.5% (Abutarbush et al. 2013).
2013: The LSD was reported in Iraq (Yousefi et al. 2017).
2013: The first reports of LSD in Turkey were in mid 2013 (Ince et al. 2016) and after that LSD virus outbreaks have expanded northwards to the west through south‐eastern Europe and to the east through the Caucasus, reaching Russia. In 2015, from Turkey the LSDV entered to Greece (August 2015) where it continued to spread westwards of Greece (EFSA 2017).
2014: The LSD was reported in Iran and estimated prevalence, cumulative mortality and case fatality were 17.9%, 3.5% and 19.7% respectively (Yousefi et al. 2017). In November 2014, the disease was also confirmed in the island of Cyprus (in the areas not under the effective control of the Republic of Cyprus) (EFSA 2017).
2015: The LSDV spread throughout the Russian Federation (Sprygin et al. 2018). The geographic pattern of spread was assessed by means of directionality, indicating a northward movement from 2015 to 2016, with a consequent East turn in 2017 through Siberia to the Far East by 2020. All cases occurred along the border with Kazakhstan (Byadovskaya et al. 2022).
2016: The first clinical cases of LSD-like lesions on lactating cows in large dairy farm in Al-Hassa Province in the eastern region of Saudi Arabia, during April 2016 (Kasem et al. 2018).
2016: In July 2016, first outbreak of LSD was reported in the rural district of Makash, Kurmangazinsky district of Atyrau the Republic of Kazakhstan (Orynbayev et al. 2021). The cases of LSD were also reported from Armenia, Bulgaria and Former Yugoslav Republic of Macedonia, Montenegro and Kosovo (Beard 2016, EFSA 2017).
2016: In 2016, the disease reappeared in Greece, close to the border with Bulgaria, in the region of Serres. Shortly, after the first outbreaks in Greece in 2015, in 2016, the disease occurred for the first time in Bulgaria, Albania, Serbia, the former Yugoslav Republic of Macedonia, Montenegro and Kosovo (EFSA 2017).
2019: LSD emerged in Bangladesh in July 2019 followed by China, India, Nepal, Bhutan, Vietnam, Hong Kong and Myanmar (Das et al. 2021). In India in August 2019, first in Odisha State and spread to other states, causing outbreaks in cattle in Odisha, Jharkhand and West Bengal States during August-December, 2019 (Singh 2019, Sudhakar et al. 2022). Similarly, in the month of early August 2019, LSD was reported from China (Das et al. 2021).
2020: In the last week of June 2020 first outbreak of LSD in cattle was reported from Morang district of Nepal and afterwards to other parts of the country (Acharya & Subedi 2020). The first outbreak of LSD was reported from Hong Kong in October 2020 (Flannery et al. 2022). The LSD outbreak was also reported from Vietnam and Myanmar during the early October and November 2020 respectively (Das et al. 2021). After Odisha and West Bengal, the outbreaks of LSD were also reported from Madhya Pradesh and Maharastra in 2020.
2021: The first LSD outbreak was detected in southern Pakistan in November 2021 and notified by the government on March 4th, 2022 (Imran et al. 2022).
2022: According to the Ministry of Fisheries, Animal Husbandry, and Dairy, India, the LSD has infected more than 20 lakhs animals and now it has spread to 251 districts across 15 states as of September 2022. Cases have been documented in Rajasthan, Gujarat, Punjab, Haryana, Uttar Pradesh, Jammu and Kashmir, Himachal Pradesh, Madhya Pradesh, Uttarakhand, Maharashtra, Goa and Andaman and Nicobar Islands (Mishra et al. 2022).
LSD-Free Countries
According to the Department of Agriculture, Water and the Environment, 2022 following are the countries which are free from LSD till 16 June 2022 (Aust. Gov. 2022).
| LSD-Free Country List (Effective as of: 16 June 2022) | ||
| Country | Country | Country |
| Australia | Austria | Belgium |
| Canada | Chile | Croatia |
| Czechia (Czech Republic) | Denmark | Estonia |
| Finland | France | Germany |
| Hungary | Iceland | Ireland |
| Italy | Japan | Latvia |
| Lithuania | Luxembourg | Malta |
| Mexico | Netherlands | New Caledonia |
| New Zealand | Norway | Poland |
| Portugal | Romania | Slovakia |
| Slovenia | Spain | Sweden |
| Switzerland | United Kingdom | United States of America |
| Vanuatu | ||
| Source: Aust. Gov. 2022 | ||
All European Union Member States are recognized free of LSD except Cyprus, Greece and Bulgaria countries (GOC 2022). Nowadays, LSD has been reported from habited continents of the globe and has raised concerns about the potential transboundary spread of the LSDV in the relevant regions and other neighboring LSDV virus-free countries. However, there are expectations of the travelling and invasion of the LSD to free neighbours countries are possible.
References
- Abraham, A. and Zissman, A., 1991. Isolation of lumpy skin disease virus in Israel. Israeli Journal of Veterinary Medicine, 46, pp.20-23.
- Abutarbush, S.M., Ababneh, M.M., Al Zoubi, I.G., Al Sheyab, O.M., Al Zoubi, M.G., Alekish, M.O. and Al Gharabat, R.J., 2013. Lumpy Skin Disease in J ordan: Disease Emergence, Clinical Signs, Complications and Preliminary‐associated Economic Losses. Transboundary and emerging diseases, 62(5), pp.549-554. [Web Reference]
- Acharya, K.P. and Subedi, D., 2020. First outbreak of lumpy skin disease in Nepal. Transboundary and Emerging Diseases, n/a. Preventive Veterinary Medicine, 102(4), pp.274-283. [Web Reference]
- Ahmed, A.M. and Dessouki, A.A., 2013. Abattoir-based survey and histopathological findings of lumpy skin disease in cattle at Ismailia abattoir. International Journal of Bioscience, Biochemistry and Bioinformatics, 3(4), p.372. [Web Reference]
- Alexander, R.A., Plowright, W. and Haig, D.A., 1957. Cytopathogenic agents associated with lumpy skin disease of cattle. Bulletin of epizootic diseases of Africa, 5, pp.489-492.
- Ali, B.H. and Obeid, H.M., 1977. Investigation of the first outbreaks of lumpy skin disease in the Sudan. British Veterinary Journal, 133(2), pp.184-189. [Web Reference]
- Ayelet, G., Haftu, R., Jemberie, S., Belay, A., Gelaye, E., Sibhat, B., Skjerve, E. and Asmare, K., 2014. Lumpy skin disease in cattle in central Ethiopia: outbreak investigation and isolation and molecular detection of the virus. Rev. Sci. Tech, 33(3), pp.877-87. [Web Reference]
- Beard, P.M., 2016. Lumpy skin disease: a direct threat to Europe. The Veterinary Record, 178(22), p.557. [Web Reference]
- Brenner, J., Haimovitz, M., Oren, E., Stram, Y., Fridgut, O., Bumbarov, V., Kuznetzova, L., Oved, Z., Waserman, A. and Garazzi, S., 2006. Lumpy skin disease (LSD) in a large dairy herd in Israel, June 2006. Israel Journal of Veterinary Medicine, 61(3/4), p.73. [Web Reference]
- Brenner, J.D., David, A., Avraham, U., Klopfer-Orgad, I., Samina, P.B. and Peleg, B.A., 1992. Experimental infection with local lumpy skin disease virus in cattle vaccinated with sheep pox vaccine. Isr. J. Vet. Med, 47, pp.17-21.
- Byadovskaya, O., Prutnikov, P., Shalina, K., Babiuk, S., Perevozchikova, N., Korennoy, F., Chvala, I., Kononov, A. and Sprygin, A., 2022. The changing epidemiology of lumpy skin disease in Russia since the first introduction from 2015 to 2020. Transboundary and Emerging Diseases, 69(5), pp.e2551-e2562. [Web Reference]
- Das, M., Chowdhury, M.S.R., Akter, S., Mondal, A.K., Uddin, M.J., Rahman, M.M. and Rahman, M.M., 2021. An updated review on lumpy skin disease: Perspective of southeast asian countries. J. Adv. Biotechnol. Exp. Ther, 4(3), pp.322-333. [Web Reference]
- Davies, F.G., 1991. Lumpy skin disease of cattle: a growing problem in Africa and the Near East. World Animal Review, 68(3), pp.37-42.
- Diesel, A.M., 1949. The epizootology of” lumpy skin disease” in South Africa.
- EFSA, 2017. Lumpy skin disease: I. Data collection and analysis. European Food Safety Authority (EFSA). EFSA Journal, 15(4):4773. [Web Reference]
- Fenner, F., Pereira, H.G. and Porterfield, J.S., 1974. International Committee on Taxonomy of Viruses, June 1974. Intervirology, 3, pp.193-198. [Web Reference]
- Flannery, J., Shih, B., Haga, I.R., Ashby, M., Corla, A., King, S., Freimanis, G., Polo, N., Tse, A.C.N., Brackman, C.J. and Chan, J., 2022. A novel strain of lumpy skin disease virus causes clinical disease in cattle in Hong Kong. Transboundary and Emerging Diseases, 69(4), pp.e336-e343. [Web Reference]
- Greth, A., Gourreau, J.M., Vassart, M., Wyers, M. and Lefevre, P.C., 1992. Capripoxvirus disease in an Arabian oryx (Oryx leucoryx) from Saudi Arabia. Journal of Wildlife Diseases, 28(2), pp.295-300. [Web Reference]
- Haig, D.A., 1957. Lumpy skin disease. Bull. Epizoot. Dis. Afr, 5(9), pp.421-430.
- Hulo, C., De Castro, E., Masson, P., Bougueleret, L., Bairoch, A., Xenarios, I. and Le Mercier, P., 2011. “Poxviridae” excerpted from ViralZone: a knowledge resource to understand virus diversity. Nucleic acids research, 39(suppl_1), pp.D576-D582. [Web Reference]
- Imran, M., Hashmi, A.H., Khalique, F. and Iqbal, M.Z., 2022. Lumpy Skin Disease Emerging Problem in Pakistan. [Web Reference]
- Ince, O.B., Çakir, S. and Dereli, M.A., 2016. Risk analysis of lumpy skin disease in Turkey. Indian Journal of Animal Research, 50(6), pp.1013-1017. [Web Reference]
- Kasem, S., Saleh, M., Qasim, I., Hashim, O., Alkarar, A., Abu‐Obeida, A., Gaafer, A., Hussien, R., AL‐Sahaf, A., Al‐Doweriej, A. and Bayoumi, F., 2018. Outbreak investigation and molecular diagnosis of Lumpy skin disease among livestock in Saudi Arabia 2016. Transboundary and emerging diseases, 65(2), pp.e494-e500. [Web Reference]
- Kitching, R.P., 2004. Current global situation of emerging infectious diseases of livestock. Food and Fertilizer Technology Center. [Web Reference]
- Kumar, S.M., 2011. An outbreak of lumpy skin disease in a Holstein Dairy Herd in Oman: a clinical report. Asian Journal of Animal and Veterinary Advances, 6(8), pp.851-859. [Web Reference]
- Kumar, S.M., 2011. An outbreak of lumpy skin disease in a Holstein Dairy Herd in Oman: a clinical report. Asian Journal of Animal and Veterinary Advances, 6(8), pp.851-859. [Web Reference]
- Le Roux, P.L., 1945. Notes on the probable cause, prevention and treatments of pseudo-urticaria and associated septic conditions in cattle. Northern Rhodesia Department of Animal Health, Newsletter, pp.1-4.
- MacDonald, R.A.S., 1931. Pseudo-urticaria of cattle. Government of Northern Rhodesia: Department of Animal Health, pp.20-1.
- MacOwan, K.D.S., 1959. Observations on the epizootiology of lumpy skin disease during the first year of its occurrence in Kenya. Bull. Epizootic Dis. of Africa, 7, pp.7-20.
- Maw, M.T., Khin, M.M., Hadrill, D., Meki, I.K., Settypalli, T.B.K., Kyin, M.M., Myint, W.W., Thein, W.Z., Aye, O., Palamara, E. and Win, Y.T., 2022. First Report of Lumpy Skin Disease in Myanmar and Molecular Analysis of the Field Virus Isolates. Microorganisms, 10(5), p.897. [Web Reference]
- Mishra, R., Gairola, V. and Bhardwaj S., 2022. Lumpy Skin Disease (LSD): An Emerging Transboundary Viral Disease. Pashudhan Praharee. October 09, 2019. [Web Reference]
- Nawathe, D.R., Gibbs, E.P.J., Asagba, M.O. and Lawman, M.J.P., 1978. Lumpyskin disease in Nigeria. Tropical Animal Health and Production, 10(1), pp.49-54. [Web Reference]
- OIE, 2017. Lumpy Skin Disease. World Organisation for Animal Health. [Web Reference]
- Orynbayev, M.B., Nissanova, R.K., Khairullin, B.M., Issimov, A., Zakarya, K.D., Sultankulova, K.T., Kutumbetov, L.B., Tulendibayev, A.B., Myrzakhmetova, B.S., Burashev, E.D. and Nurabayev, S.S., 2021. Lumpy skin disease in Kazakhstan. Tropical Animal Health and Production, 53(1), pp.1-7. [Web Reference]
- Prydie, J. and Coackley, W., 1959. Lumpy skin disease: tissue culture studies. Bulletin of epizootic diseases of Africa, 7(1), pp.37-50.
- Roy, P., Purushothaman, V., Sreekumar, C., Tamizharasan, S. and Chandramohan, A., 2008. Sheep pox disease outbreaks in Madras Red and Mechery breeds of indigenous sheep in Tamilnadu, India. Research in Veterinary Science, 85(3), pp.617-621. [Web Reference]
- Salib, F.A. and Osman, A.H., 2011. Incidence of lumpy skin disease among Egyptian cattle in Giza Governorate, Egypt. Veterinary world, 4(4):162-167. [Web Reference]
- Schoch, C.L., Ciufo, S., Domrachev, M., Hotton, C.L., Kannan, S., Khovanskaya, R., Leipe, D., Mcveigh, R., O’Neill, K., Robbertse, B. and Sharma, S., 2020. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford), 2020. [Web Reference]
- Shimshony, A. and Economides, P., 2006. Disease prevention and preparedness for animal health emergencies in the Middle East. Revue scientifique et technique-Office international des épizooties, 25(1), p.253. [Web Reference]
- Singh, R.K., 2019. OUTBREAK OF LUMPY SKIN DISEASE (LSD) IN CATTLE IN CHHOTANAGPUR PLATEAU REGION (INDIA). Pashudhan Praharee. August 31, 2019. [Web Reference]
- Sprygin, A., Artyuchova, E., Babin, Y., Prutnikov, P., Kostrova, E., Byadovskaya, O. and Kononov, A., 2018. Epidemiological characterization of lumpy skin disease outbreaks in Russia in 2016. Transboundary and emerging diseases, 65(6), pp.1514-1521. [Web Reference]
- Sudhakar, S.B., Mishra, N., Kalaiyarasu, S., Jhade, S.K. and Singh, V.P., 2022. Genetic and phylogenetic analysis of lumpy skin disease viruses (LSDV) isolated from the first and subsequent field outbreaks in India during 2019 reveals close proximity with unique signatures of historical Kenyan NI‐2490/Kenya/KSGP‐like field strains. Transboundary and Emerging Diseases, 69(4), pp.e451-e462. [Web Reference]
- Tageldin, M.H., Wallace, D.B., Gerdes, G.H., Putterill, J.F., Greyling, R.R., Phosiwa, M.N., Al Busaidy, R.M. and Al Ismaaily, S.I., 2014. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Tropical animal health and production, 46(1), pp.241-246. [Web Reference]
- Tageldin, M.H., Wallace, D.B., Gerdes, G.H., Putterill, J.F., Greyling, R.R., Phosiwa, M.N., Al Busaidy, R.M. and Al Ismaaily, S.I., 2014. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Tropical animal health and production, 46(1), pp.241-246. [Web Reference]
- Thomas, A. D., Robinson E. M. and Alexander R. A., 1945. Lumpy skin disease: Knopvelsiekte. Onderstepoort, Division of Veterinary Services, Veterinary Newsletter No. 10.
- Thomas, A.D. and Mare, C.V.E., 1945. Knopvelsiekte. Journal of the South African Veterinary Association, 16(1), pp.36-43. [Web Reference]
- Tulman, E.R., Afonso, C.L., Lu, Z., Zsak, L., Kutish, G.F. and Rock, D.L., 2001. Genome of lumpy skin disease virus. Journal of virology, 75(15), pp.7122-7130. [Web Reference]
- Tuppurainen, E., Alexandrov, T. and Beltrán-Alcrudo, D., 2017. Lumpy skin disease field manual – A manual for veterinarians. FAO Animal Production and Health Manual No. 20. Rome. Food and Agriculture Organization of the United Nations (FAO). 60 pages. [Web Reference]
- ViralZone, 2014. Capripoxvirus. Swiss Institute of Bioinformatics. [Web Reference]
- Von Backstrom, U., 1945. Ngamiland cattle disease: preliminary report on a new disease, the etiological agent being probably of an infectious nature. Journal of the South African Veterinary Association, 16(1), pp.29-35. [Web Reference]
- Wainwright, S., El Idrissi, A., Mattioli, R., Tibbo, M., Njeumi, F. and Raizman, E., 2013. Emergence of lumpy skin disease in the Eastern Mediterranean Basin countries. FAO Empres Watch, 29, pp.1-6. [Web Reference]
- Weiss, K.E., 1968. Lumpy skin disease virus. In Cytomegaloviruses. Rinderpest Virus. Lumpy Skin Disease Virus (pp. 111-131). Springer, Berlin, Heidelberg. [Web Reference]
- Woods, J.A., 1988. Lumpy skin disease—a review. Tropical animal health and production, 20(1), pp.11-17.
- Yeruham, I., Nir, O., Braverman, Y., Davidson, M., Grinstein, H., Haymovitch, M. and Zamir, O., 1995. Spread of lumpy skin disease in Israeli dairy herds. Veterinary Record, 137, pp.91-91. [Web Reference]
- Yousefi, P.S., Mardani, K., Dalir‐Naghadeh, B. and Jalilzadeh‐Amin, G., 2017. Epidemiological study of lumpy skin disease outbreaks in North‐western Iran. Transboundary and emerging diseases, 64(6), pp.1782-1789. [Web Reference]
- GOC, 2022. Lumpy Skin Disease – Countries that Canada recognizes as being free from the disease. Government of Canada. Assessed on September 24, 2022. [Web Reference]
- Gov. 2022. LSD-Free Country List. Department of Agriculture, Water and the Environment, Australian Government. Assessed on September 24, 2022. [Web Reference]