An update on Equine Piroplasmosis : A potential threat to Zoonosis
Equine Piroplasmosis (EP) is a tick-borne disease that affects horses, donkeys, mules and zebras. Ticks ingest blood from the infected equine and transfer the parasite to an uninfected equine by feeding on the host, spreading the disease through blood contact. Because the disease is spread through blood, EP can also be transmitted through blood transfusion when the source of blood is an infected horse, previously used needles or syringes and other skin penetrating instruments that are contaminated with blood and have not been adequately sanitized between horses (i.e. dental, tattoo and surgical equipment). Moreover, EP is very infectious and there is a threat for emergence of new zoonosis. Anti-protozoal drugs are only the hope for clearance of parasite due to unavailability of vaccines. Proper sanitation and testing might reduce some chance of infection.
INTRODUCTION
Equine piroplasmosis (EP) is a tick-borne disease of equines that infects horses, mules, donkeys and zebra. It is caused by the intraerythrocytic protozoan parasites Babesia caballi and Theileria equi of the Order Piroplasmida. This agent does not survive outside its hosts and can only be transmitted through a tick vector. Infected animals may remain carriers of these blood parasites for long periods and act as sources of infection for other ticks. The introduction of carrier animals into areas where competent tick vectors are prevalent can lead to an epizootic spread of the disease. Blood infected with causative parasites of piroplasmosis and associated vectors (i.e. ticks and mechanical vectors) are the source of infection. Moreover, infected animals may remain carriers of these blood parasites for long periods and act as sources of infection for tick vectors .
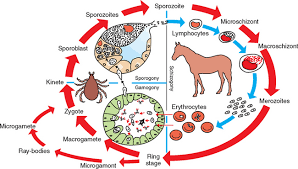
LIFE CYCLE, TRANSMISSION AND OCCURRENCE
The life cycle of a tick includes four stages (egg, larva, nymph, and adult) and transmission of the parasites can occur in three distinct forms: (1) intrastadially (between two ticks), (2) transtadially (between two life stages of tick), or (3) transovarially (parent to offspring). Babesia sporozoites invade red blood cells (RBCs) and transform into trophozoites which grow and divide into two rounds, oval or pearshaped merozoites which, in turn, are capable of infecting new RBCs and the division process is then repeated. Theileria equi sporozoites inoculated into horses via a tick bite invade the lymphocytes and these intralymphocytic forms undergo development and eventually form Theileria-like schizonts. Further, merozoites released from these schizonts invade RBCs and transform into trophozoites which grow and divide into pear-shaped tetrad (‘Maltese cross’) merozoites. Twelve species of Ixodid ticks in the genera Dermacentor, Rhipicephalus and Hyalomma have been identified as transstadial vectors of B. caballi and T. equi, while eight of these species were also able to transmit B. caballi infections transovarially. Theileria equi develop in salivary glands of tick vector and not found in other tick organs. It is not transmitted transovarially from egg to larva. Transmission is also possible through mechanical vectors contaminated by infected blood (e.g. contaminated needles).
The presence of competent tick vectors and infected horses within the same area does not always lead to further infection or disease. Many factors must be considered including season, climate, host specificity, and the particulars of the competent tick’s life cycle. These ticks are found in tropical, subtropical, and some temperate climates. According to the Office International des Epizooties/The World Organization for Animal Health (OIE), most of the equid-inhabited regions of the world are considered endemic for infection and disease.
CLINICAL SIGNS
Incubation period of equine piroplasmosis associated with T. equi is 12 to 19 days and approximately 10 to 30 days when caused by B. caballi. The clinical signs of equine piroplasmosis are often nonspecific, and the disease can easily be confused with other similar hemolytic conditions presenting fever, anaemia and jaundice. In general, infection with T. equi results in more severe clinical disease than B. caballi and signs and severity can vary significantly from one region to another. Piroplasmosis can occur in per-acute, acute, subacute and chronic forms. Most animals in endemic areas survive infection. Per-acute form is rare form of disease with only clinical observation in dead animals. Acute form is most common form of disease cases characterised by fever that usually exceeds 40°C, reduced appetite and malaise, elevated respiratory and pulse rates, congestion of mucous membranes, production of a dark red urine, faecal balls that are smaller and drier than normal and affected animals may appear unthrifty, anemic and/or icteric. Subacute form is similar to acute form but accompanied by weight loss in affected animals, intermittent fever, mucous membranes vary from pale pink to pink, or pale yellow to bright yellow; petechiae and/orecchymoses may also be visible on the mucous membranes; normal bowel movements may be slightly depressed and the animals may show signs of mild colic. Chronic cases usually present nonspecific clinical signs such as mild inappetence, poor performance and a drop-in body mass. Mild anaemia may be present and the spleen might be enlarged on rectal palpation (Bruning, 1996).
DIAGNOSIS
Routine laboratory analyses may aid in confirming clinical findings. Most horses regardless of clinical syndrome exhibit some degree of anaemia characterized by decreased packed cell volume, hemoglobin, and erythrocyte count. In these animals, thrombocytopenia is common and clotting times can be prolonged or normal (Zobba et al, 2008). T. equi infection in equines can be diagnosed by number of different methods including Giemsa stained blood smears, in vitro culture method, ELISA and PCR. Light microscopy can be used to identify the organisms within the erythrocytes during the acute stage of infection. A thin blood smear can reveal organisms, but smears must be thoroughly examined because even during severe infection, the percent parasitemia remains quite low. In cases of chronic or inapparent infection, parasite numbers are too low for reliable detection on blood smears. Several serological tests were developed to increase diagnostic sensitivity especially in those carrier horses exhibiting no clinical signs. These tests include the complement fixation test (CFT), indirect immunofluorescence assay (IFA), Western blot (WB), and competitive enzyme-linked immunosorbent assay (cELISA). The CFT depends on activation of complement during specific interaction of antibody and antigen. Infected horses seroconvert on the CFT approximately 8 to 11 days after infection with titers beginning to decline at 2 to 3 months. The CFT is a very specific test yet lacks sensitivity in chronic or inapparent phases of infection mainly because some antibodies produced during these phases of infection do not fix complement, therefore should only be used in acute cases of infection. The IFA is considered to be more sensitive than the CFT during chronic infection. However, serum is diluted to improve specificity in IFAT but it results in compromise with decreased sensitivity. Experimentally infected T. equi and B. caballi horses are positive on the IFA at 3 to 20 days post-infection (Weiland, 1986). The cELISA, which detects antibody responses to Equine merozoite surface antigens (EMA) 1 and 2, is validated for detection of antibodies against numerous isolates of T.equi found globally (Knowles et al, 1992). The B. caballi cELISA is also routinely used for detection of chronic infection or inapparent carriers (Kappmeyer et al, 1999). A WB test is also under validation to be used as a means of clearance confirmation in T. equi– infected horses treated with imidocarb diproprionate (ID). PCR relies on the amplification and detection of parasite DNA isolated from the peripheral blood of an infected horse. It is an exquisitely sensitive test which can detect a positive animal with T. equi infection having parasitemia as low as one parasite (Nicolaiewsky, 2001). Because of the sensitivity of PCR, it is currently being used as one of several tests to confirm clearance of T. equi horses.
PREVENTION AND CONTROL:
Prevention and control strategies differ tremendously between endemic and nonendemic countries. Prevention is virtually impossible in endemic regions and naivety is not desired because infection provides a degree of protection. In non-endemic countries, the foundation of prevention is supervised on import and testing of horses from endemic areas (by IFA or ELISA as described above). Non-endemic nations that border endemic nations cannot completely prevent introduction of ticks so diligent measures must be taken to reduce horses’ contact with ticks by using repellents, acaricides and regular inspection of animals and premises. For controlling and eradication of the tick vector nearby vegetation should be removed that could harbour ticks. Any detected EP-positive animals should be quarantined from surrounding horses and vectors. For the prevention of EP, no vaccine is available currently, therefore antiprotozoal agents can be used for temporary clearance of T. equi from carriers.
CONCLUSION
Equine piroplasmosis has a major effect on the horse industry around the world, specially sports industry. If infected horses are allowed in for sporting events, the horses should be examined thoroughly and then carefully monitored and quarantined appropriately. Horse owners are encouraged to ask a veterinarian about the disease and preventive measure. Enhanced control through surveillance of equine and vector populations is of paramount importance. Practicing stringent hygiene reduces opportunities for the transfer of infected blood. Moreover, infected samples should be handled with proper care as it is highly zoonotic disease. By taking some action to combat the disease, it would helpful for achievement of OIE prime goal i.e., ‘protecting animals, preserving human future’.
Compiled & Shared by- This paper is a compilation of groupwork provided by the Team, LITD (Livestock Institute of Training & Development)
Image-Courtesy-Google
Reference-On Request