Antibiotic Residues: A Global Health Hazard!
Abstract:
Antibiotics are used to treat disease and improve animal production. These antibiotics might result in deposition of residues in meat, milk and eggs which are not permitted in food intended for human consumption. There are many factors influencing the occurrence of residues in animal products such as drug’s properties and their pharmacokinetic characteristics, physicochemical or biological processes of animals and their products. The use of antibiotics is necessary in the prevention and treatment of animal diseases. Moreover, these antibiotics also improve the performance of growth and feed efficiency, synchronizing the reproductive cycle and breeding performance. However, the residues and their breakdown products have several side effects on the human body and, in a broader sense, on the environment. In relation to the human body, the frequency of mutations is increased, the bone marrow is damaged (chloramphenicol), and the reproductive organs of humans are affected. Carcinogenic effects have been found with antibiotics such as sulfamethazine, oxytetracycline, and furazolidone that are freely available for sale.
Introduction: Veterinary antibiotics are extensively applied for therapeutic and prophylactic infections of different animals. Antimicrobials are the dominant class of veterinary drugs used after 1950s to treat bacterial infectious diseases in animals. However, after the use of veterinary drugs in food-producing animals, parent compounds and their metabolites may accumulate in the products of animal origin. The normal use of veterinary drugs is acceptable in risk analysis by dietary intake assessment. But, unreasonable use of veterinary drugs due to the lack of scientific knowledge and the blind pursuit of economic benefits by husbandry personnel may lead to the existence of high drug residue in animal-derived food products . The existence of veterinary antibiotic residues in animal products such as milk and meat may cause allergies in humans, and in the extensive and long run may facilitate the development of resistant pathogens. The existence of resistant bacterial strains produces severe health consequences on the human body . These reasons make it important to Effectively control antibiotic residues in animal-derived food products, and therefore, regulatory authorities have enacted maximum residue limits (MRLs) for a number of anti-infective agents in milk and meat .during the production processing and storage of milk and milk products. Direct contamination of milk may occur from air and water during processing,storage and transportation. Besides feed given to animals is also source of indirect contamination. Man will be the ultimate consumer of these antibiotic residues.There are some causes of miscellaneous uselike lack of awareness, lack of extension activities,inadequate literature supplied by manufacturers, lackof safer drugs and exploitation of more production and profit from animals.FDA prohibits the extra label use of chloramphenicol, furazolidone, nitrofurazone,sulphonamide drugs, and flouroquinolones in lactatinganimals.
Use of antibiotics in foods: Antibiotics are widely used in animals for many advantages covering carcass quality, promotion of growth, animal health, and cost-effective production. Antibiotics are the commonest drugs and are widely being used in animals as prophylactic and therapeutic agents for the management of infectious diseases. These antibiotics have played an important role in the prevention and cure of certain important infections caused by Escherichia coli, Campylobacter fetus, Enterococcus, Leptospira, Streptococcus suis, and Salmonella. Antibiotics enhance growth rate by altering the motility of the gut, by thinning the mucous layers in the gut, by decreasing waste nutrients, immune system activity, and formation of toxins, and by providing favorable conditions for beneficial intestinal microbes to destroy harmful bacteria . The body weight of animals increases up to 4–5% that receive antibiotics compared to those which are grown in the absence of these drugs.. In veterinary medicine, several groups of antibiotics are used for these purposes, for example, lincosamide, aminoglycosides, ansamycins, and glycopeptides, β-lactams i.e. cephalosporin and penicillin, trimethoprim, Sulfonamides, quinolones, nitrofurans, tetracycline. Few drugs or and their combinations are used as prophylaxis for different plant diseases for example streptomycin- oxytetracycline is used to control halo blight disease in beans and few other bacterial diseases in tomatoes, potatoes, cherries, tobacco, and peppers. Several drugs are used to inhibit trees from bacterial diseases such as aminoglycosides and tetracyclines .Antibiotics are used in the preservation of foods, particularly food of animal origin, such as poultry and fish. The antibiotics are added to water at a concentration of 5 to 40 ppm and poultry meat is dipped into treated water for chilling purposes during production.
Techniques used for Detection and Analysis of Drug Residues:
1.ELISA
2.HPLC
- Liquid chromatography
4.Gas chromatography
5.paper chromatography.
Pathological Effects produced by Antibiotic Residues in Food:
-Transfer of antibiotic resistant bacteria to the human.
– Immuno pathological effects
-Autoimmunity
– Carcinogenicity (Sulphamethazine,Oxytetracycline, Furazolidone)
– Mutagenicity
– Nephropathy (Gentamicin)
– Hepatotoxicity
-reproductive disorder
-bone marrow toxicity (chloramphenicol)
-allergy(penicillin)
-Teratogenic effect :Any chemical agent or drug that produces a harmful effect on a fetus or embryo during gestation is termed a teratogen. As a result, congenital disorders that influence functional, as well as structural integrity, take place . For example, when benzimidazole and anthelmintic are administered at an early stage of pregnancy they produce toxic effects on the embryo, furthermore, the drug of oxfendazole i.e. benzimidazole has a mutagenic effect. Enrofloxacin; a fluoroquinolone antibiotic that inhibits the bacteria by targeting the DNA gyrase has shown to be teratogenic for the embryos of rabbits and rats.
Public health aspect : antibiotic residues in milk are a major problem for public health as milk and meat are commonly consumed throughout the world by children, youth and adults. Antibacterial residues may pose hazards to human pharmacology, toxicology, microbiology, and immunopathology. Acute and chronic adverse effects of antibiotics residues are transfer of antibiotic resistant bacteria to the human, autoimmunity, carcinogenicity due to sulphamethazine and oxytetracycline, mutagenicity, nephropathy due to gentamicin, hepatotoxicity, reproductive disorders, chloramphenicol induced bone marrow toxicity, allergy reactions due to penicillin. These hazards can be classified as direct-short-term hazards and indirect-long-term hazards in up to two types, depending on the duration of residue exposure and the timing of health effects. Direct health risks include the health impacts triggered by the excretion of the drug in milk, as an instance of which the beta-lactam group of antibiotics, irrespective of their low milk concentration, triggers an allergic hypersensitive reaction in the sensitized person instantly after consumption, while chronic toxic impacts with extended exposure to small concentrations of antibiotics include carcinogenicity, teratogenicity, reproductive effects, development of antibiotic resistance bacteria in treated animals and destruction of normal human flora in the gut. Chronic OTC exposure includes changes in blood such as leucocytosis, atypical lymphocytes, lung congestion, granulocyte toxic granulation, and purpura thrombocytopenia, and brown teeth discoloration. Antibacterial agents such as tetracyclines, nitrofurans, and sulfonamides are used in cattle feed as feed additives that can excrete in milk and are sometimes linked with human toxicology.
The insufficiency of attention among farmers and breeders concerning the withdrawal periods (WDPs) and health risks related to the presence of AMRs in different types of food, especially in developing countries, is globally recognized . Additionally, failure to follow the instructions of antibiotics manufacturers also accounts for residue occurrence in meat . The phrase withdrawal period (WDP) is often used more broadly to describe the time that must pass after the last given dose of veterinary medication and before the slaughter or production of food from the treated animal to ensure that the food does not contain levels of the medicine that exceed the maximum residue limit (MRL) .
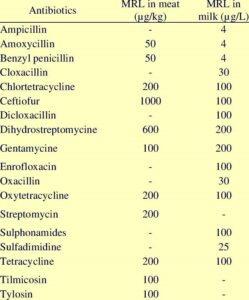
Several organisations such as the Food and Agriculture Organization (FAO) and the European Union (EU) have set tolerance or maximum residue limits (MRL) for antibiotic residues in food-stuffs derived from animals according to the regulations governing the maximum limits for veterinary medicine.The word MRL may be characterized as the maximum concentration of marker residue resulting from the use of a veterinary drug expressed as new weight components like parts per million (ppm) or parts per billion (ppb) legally allowed or acknowledged as acceptable in or on food. The MRL of a compound is based on the acceptable daily intake (ADI). The ADI is a rough estimate of the quantity of a veterinary drug given on a body weight basis that can be ingested daily by a person without significant toxicological health danger throughout a lifetime and can be regarded the safety standard for that compound. MRL of antibiotics like:
Residues prevention:
The first step in residue prevention is to make individuals and organizations aware of the problem through education by veterinary personnel, organizations, and literatures and governmental agencies.
– Rapid screening procedures for the analysis of antibiotic residues and instant grading and prohibition of food containing antibiotics more than MRL.
– Processing of milk help for the inactivation of antibiotics. Refrigeration causes disappearance of penicillin. In pasteurization most of antibiotics will loose activity.
– Use of activated charcoal, resin and UV irradiation also help for antibiotic inactivation.
– Irrational use of antibiotics in field veterinary practices should be avoided.
– Development of simple and economic field test to identify drug residue in edible animal products.
– Ethno-veterinary practices may be promoted.
– Nation wide monitoring and periodic surveillance of microbial residue in edible tissues and milk.
Follow the five R’s to prevent drug residues:
1.Relationships: Develop good relationships with people involved in the process.Establish a good Veterinarian-Client-Patient Relationship (VCPR).Review veterinary recommendations with employees and family members who work on the farm.
2.Responsible use: Use and handle veterinary drugs responsibly..Minimize the use of veterinary drugs to times when they are medically necessary.
3.Recordkeeping: Maintain good records to document treatment.Maintain a recordkeeping system to document all treatments given.Identify the animal before it is treated.Keep treatment records for at least 3 years.
4.Respect withdrawal times and usage limitations:Use only veterinary drugs that are approved by the FDA for use in the species and animal class you are treating.
5.Remove doubt:Test milk from treated, fresh and newly purchased cows for drug residues before commingling into the bulk tank.
The Drug Residue Prevention Program :The FDA awarded the Minnesota Department of Agriculture (MDA) funds to develop an education and outreach program to help prevent drug residues in milk and meat and to promote antibiotic stewardship in livestock. Producers can visit the DRPP website for more information on DRPP, what the program does, and to access their library of resources.
Being proactive is the best way to prevent future residues and signing up for an on-farm visit with an outreach veterinarian is a great place to start.
Conclusion: Although the specific regulations are limited due to the lack of consensus on the safer concentrations of antibiotic residues in the environment concerning the development of resistance, The WHO emphasizes the policies for the improvement of pharmaceutical waste management and to minimize the antibiotic residues in the environment. Despite the new medicines in the USA and Europe need environmental risk assessments by considering the ecological impacts of drugs, the medicines already available in the market have not undergone these valuations. These assessments do not address the potential of the drug in resistance implications, rather emphasize the ecological toxicity. The extensive use of antibiotics in animal feed for growth promotion poses a significant threat to public health due to its impact on the development of multidrug-resistant bacterial strains. The stringent control must be adapted to avoid the excessive use of these agents accompanied by the development of alternative measures to safeguard human health and to keep the available antibiotics effective for future clinical implications. The continuous surveillance of antimicrobial resistance in the human, zoonotic and environmental bacteria is a precondition to understanding this phenomenon that can provide risk assessment data for the evaluation and implementation of targeted interventions. Awareness and communication at national and international levels are necessary for the rational use of antimicrobials in the food chains. These relevant target audiences must be identified including the decision-makers, agriculture, health and veterinary professionals, media, and the public, and should be informed with evidence-based information for their guidance, decisions, and choices. The knowledge gaps still exist in the precise understanding of antibiotic resistance and its implications in food safety. The studies must focus on the quantitative analysis of the disease burden caused by resistant bacteria. These studies will further contribute to assessing the magnitude of the problem and will assist in the risk assessment the designing cost-effective protocols to counteract this menace.
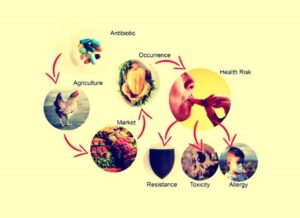
Fig.:Footprint left by antibiotic residue in food.
Acknowledgement:
I would like to express my sincere gratitude to Dr.Biju Borah, PhD Assistant Professor, Department of Veterinary & Animal Husbandry Extension Education, Lakhimpur College of Veterinary Science, for giving me this opportunity to study and write an article on the above topic.
WRITTEN BY:
Amrita Das
Third proffessional year, BVSc & AH
Lakhimpur College of Veterinary Science
Joy Hing, North Lakhimpur, 787051
References:
https://www.sciencedirect.com/science/article/pii/S1872203218301896
https://www.researchgate.net/publication/361814463_Antimicrobial_Residues_in_Meat_and_Meat_Products
https://www.mdpi.com/2079-6382/10/5/534#
https://images.app.goo.gl/KYrec37pMN13Trui7
https://images.app.goo.gl/rvVZUcSRYXseTfrB6
https://images.app.goo.gl/4U2S18mALJe2h3Jz6