Application of Immobilized Enzyme in Dairy Products
Enzymes are complex proteins which act as catalysts to accelerate the chemical reactions of living cells and bring specific chemical changes for converting specific set of reactants or substrates into specific products, without being changed themselves. These are not permanently modified by their participation in reactions and have a great specificity. In dairy industry, the use of enzymes, particularly exogenous enzymes are not fully exploited and limited to a few major and some minor applications. Enzymes play important role in the preparation of certain dairy products like cheeses, yoghurt etc., by improving texture, flavor and bringing about desirable changes in the product. Lipolytic and proteolytic enzymes can accelerate the production of flavor compounds. Successful use of preparations containing these enzymes is complicated by the need to attain a satisfactory balance among the various enzymes involved in the cheese ripening process.
“Immobilized enzymes” refer to an enzyme that has been confined or localized so that it can be reused continuously. The immobilized enzyme can be a free enzyme, cell or an organelle. There are many advantages of immobilized enzyme over simply enzyme like ability to be confined to a place, predetermined space, etc. in this immobilized form, the enzyme can be repeatedly and continuously used.
Introduction
In dairy industry, the use of enzymes, particularly exogenous enzymes are not fully exploited and limited to a few major and some minor applications. Enzymes play important role in the preparation of certain dairy products like cheeses, yoghurt etc., by improving texture, flavor and bringing about desirable changes in the product. Lipolytic and proteolytic enzymes can accelerate the production of flavor compounds. Successful use of preparations containing these enzymes is complicated by the need to attain a satisfactory balance among the various enzymes involved in the cheese ripening process.
An immobilized enzyme is an enzyme that is physically attached to a water-insoluble matrix, supporting material, or carrier. The immobilized enzyme is unable to move due to its linkage to the support or matrix, and its phase difference from substrates and products. Carriers, matrixes, or supporting materials frequently used are: ceramics, spongy glass, cellulose, sand, synthesized polymers, charcoal, metal oxides, stainless steel and polymeric gel. Today, in many cases immobilized enzymes have revealed highly efficient for commercial uses. They offer many advantages over enzymes in solution, including economic convenience, higher stability, and the possibility to be easily removed from the reaction mixture leading to pure product isolation. An immobilized enzyme is, therefore, attached to an inert, organic, or inorganic or insoluble material, such as calcium alginate or silica. Furthermore, the attachment of an enzyme to a solid support can increase its resistance to various environmental changes such as pHor temperature.
Immobilization of Enzymes
It is always cost-effective to use the enzymes more than once. Most of the enzymes are in solution with the reactants and/or products and hence it is difficult to separate them after completion of chemical reaction. In order to reuse the enzymes again after separation from the products during chemical reaction, it is necessary to employ techniques that are helpful in attaching the enzyme to the reactors. This idea has led to the employment of immobilization techniques for enzymes. The concept of enzyme immobilization was first evolved, when difficulties were experienced during the use of crude enzyme preparations in the production of wine, cheese or in tanning. The phenomenon of immobilization of enzyme on a support, was first reported by J.M. Nelson and E.G. Griffin in 1916. They reported the adsorption (immobilization) of invertase on charcoal/alumina without loss of activity. However, the technique of enzyme immobilization could be established only after a lapse of about 40 years, in 1954- 1961 when many researchers developed relevant procedures and the equipment’s.
In simple terms “immobilized” means unable to move or stationary. An immobilized enzyme is an enzyme which is attached to an inert, insoluble material such as calcium alginate over which a substrate is passed and converted to product. This technique has revolutionized the prospects of enzyme application in industry.
Immobilization is defined as the imprisonment of a biocatalyst in a distinct phase that allows exchange with, but is separated from, bulk phase in which substrate, effectors, inhibitor molecules are dispersed and monitored.
Immobilized enzyme is physically entrapped or covalently bonded by chemical means to an inert and usually insoluble matrix, where it can act upon its natural substrate. The matrix is usually a high molecular weight polymer such as polyacrylamide, carrageenan, chitin, cellulose, starch, glass beads, etc.
Advantages:
- Immobilization allows separation of enzymes from the products after completion of chemical reaction and thus can be reused or recycled.
- Immobilized enzymes have ability to bind to a matrix, by which it typically possess greater resistance to change in pH and temperature and have operational stability than the soluble form of the enzyme.
- Reaction mixture or products specifically contain only solvent and reaction products and so more or less do not require complex purification as the processed product is not contaminated with the enzyme.
- Immobilization improves the efficacy and efficiency of an enzyme.
- Certain manipulations of chemical reactions are better possible with immobilized enzymes
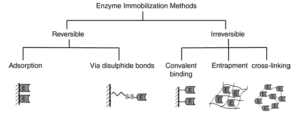
Methods of immobilization
Enzyme or cell can be immobilization can be done in various methods. Mainly classified as i) Bonding ii) Physical entrapped. The method to be adopted greatly influences the property of result product. Selection of method of immobilization depends upon the process specifications for the catalyst including such parameters like overall catalytic activity, effectiveness of the catalyst utilization, deactivation, regeneration characteristic and cost.
The Traditional Methods of Enzyme Immobilization can be classified as follows:-
1.Carrier binding
In this method, the enzymes are bound to water in-soluble carrier molecules. Based on the technique of binding, this is further divided into :-
(a)Physical adsorption:-This is the immobilization of an enzyme on the surface of water-insoluble carriers.
(b)Ionic binding: – This process involves ionic binding of the enzyme to water-insoluble carriers containing ion-exchange residues.
2.Covalent binding
This is based on the binding of enzymes and water-insoluble carriers by the formation of covalent bonds between the enzyme and the support matrix. e.g. Glutaraldehyde
3.Cross linking
This is the process of intermolecular cross-linking of enzymes by bi-functional or multi- functional reagents.
4.Entrapment
Here enzyme is trapped in insoluble beads or microspheres i.e. incorporating enzymes into the lattices of a semi-permeable gel or enclosing the enzymes in a semi-permeable polymer membrane.
Whole Cell Immobilization
The term “immobilized cells” refers to keeping the cell in one place. Generally, in a reaction environment, cells are floating around in nutrient liquid whereas in cell immobilization, the cells are trapped or stuck to a sticky surface while nutrient flows over them. This method is more appropriate and useful when there is a need for using or when a series of enzymes are required in the process.
Whole cell immobilization was defined as “the physical confinement or localization of intact cells to a certain region of a space with preservation of some desired catalytic activity”, and is a process that often mimics what occurs naturally when cells grow on surfaces or within natural structures.
Advantages of whole-cell immobilization
- Enzyme isolation and purification steps are not required
- Show the higher stability in the catalytic power and enzyme activity,
- Have multivariate enzyme applications, eliminating the need for immobilization of multiple enzymes
- Intracellular enzymes need not be extracted prior to the reaction; they may be used directly
- The end products can be recovered in a simple manner.
- The technique is cost effective.
Disadvantages
- Low productivity
- Lower resistance
- Limitation of mass transfer
- Problems with degradation of product
- Byproducts are formed due to lysis of cells or toxic metabolites
The release of cells growing in the peripheral layer of highly colonized gel beads can be used to efficiently produce biomass in the bulk liquid medium. This cell release activity can be used for producing single or mixed strain cultures and to continuously inoculate food liquids to process fermented foods such as fermented milk products, bulk lactic starter cultures, mass Probiotic cultures and pre-fermentation of milk. The immobilized cell technology can be used for production of different metabolites and functional ingredients from LAB using this low value whey permeate containing high lactose and mineral contents, used as a culture medium for the production of lactic starter cultures or metabolites .Lactic acid production, Exo polysaccharide production and Bacteriocin production are few more applications. The other food industries like in beer and wine making are the best examples of industrial exploitation of immobilized cell technology
ENZYMES COMMONLY USED IN INDUSTRY
LIPASE- Lipases (triacylglycerol acylhydrolase) act on carboxylic ester bonds, and require no cofactor. Long-chain fatty acids are the natural substrates for lipase. Lipases are of interest for industry because of their natural function of hydrolyzing triglycerides into diglycerides, monoglycerides, fatty acids, and glycerol.
AMYLASE- Enzymes which are capable of hydrolyzing the α-1,4-glucosidic linkages of starch are called amylases (Vihinen&Mantsiila, 1989). Although amylases are found in plants and animals, microbial amylases are most common in industry. α-Amylase (EC 3.2.1.1) and glucoamylase (EC 3.2.1.3) are two major amylases.
PECTIC ENZYMES- These enzymes can hydrolyze the long and complicated molecules named pectins that are structural polysaccharides in the plant cell and maintain integrity of the cell wall. . Pectic substances are high molecular weight (30,000–300,000 Da), negatively charged complex polysaccharides, with a backbone of galacturonic acid residues linked by α-1,4- linkages (Kashyap et al., 2001). The American Chemical Society has categorized pectic substances into four main groups. The first group, called protopectins, are water-soluble pectic substances composed of pectin or pectic acid. The second group is pectic acid and polymers of galacturonans that contain negligible amounts of methoxyl groups. Pectinic acid, the third group, is the polygalacturonan chain with various amounts of methoxyl groups (0–75%). Pectin is the last group and defines the mixture of differing compositions of galacturonate units esterified with methanol.
LACTASE- The enzyme β-galactosidase is also known as lactase. It is obtained from microorganisms, plants, and animals. It is used for the hydrolysis of the disaccharide sugar lactose present in milk and whey.
PROTEASES- Proteases are protein-degrading enzymes and catalyze the cleavage of peptide bonds in the proteins. Proteases are classified according to their catalytic action into endopeptidases and exopeptidases. Proteases are found in plants, animals, and microorganisms.
OXIDOREDUCTASE-Oxidoreductases are another group of enzymes that catalyze the oxidation/reduction reaction. Oxidoreductases play a crucial role in foods in terms of taste, texture, shelf life, appearance, and nutritional value. Lipoxygenase, Lactoperoxidase, Polyphenol oxidase, peroxidase, Horseradish peroxidase, Lactoperoxidase, Catalase etc are commonly used in industries.
APPLICATIONS OF IMMOBILIZED ENZYME IN FOOD SECTOR
Microbial enzymes have been used in the food industry for centuries. They also had applications in the leather industry, such as using dung for preparation of hides (Underkofler et al., 1958). In the 1930s, enzyme technology was used for the first time in the food industry, to clarify fruit juice.
Dairy Industry:
Lipases are commonly used in the dairy industry to hydrolyze milk fat, and current applications of lipases in the dairy industry include cheese ripening, flavor enhancement, manufacturing cheese-like products, and lipolysis of cream and butterfat . Cheese texture is dependent on fat content so lipases that release short-chain fatty acids (C4 and C6) develop the sharp and tangy flavor, whereas release of medium-chain fatty acids (C12 and C14) causes a soapy taste in the product (Hasan et al., 2006). Lipases are also used for enzyme-modified cheeses (EMC) to liberate fatty acids at sn-1 and sn-3 positions on the glycerol backbone (Houde et al., 2004). EMC find applications in the food industry to add cheese flavor to salad dressings, dips, soups, sauces, and snacks. Proteases have a broad application in the food industry. In the dairy industry, milk-coagulating enzymes (animal rennin, microbial coagulants, engineeredchymosin) are extensively used for cheese making. Chymosin has advantages over animal rennin due to its specific activity and availability. The protease-producing GRAS microorganisms are Mucormichei, Bacillus subtilis, and Endothiaparasitica. Oxidoreductases are employed in the pasteurization of eggs and cheese by H2O2, desugaring of eggs prior to spray drying, preservation of raw milk, and elimination of cooked flavor of UHT (ultra high temperature) pasteurized milk. The most commonly used lactases for immobilization are obtained from E. coli and A. niger. The lactase enzyme immobilized on Teflon stirring bars that are coated with a polymer polyisocyanate was stable up to pH8.75. It can be used continuously for 137.6h without appreciable losses in activity. d-Tagatose is a monosaccharide naturally present in dairy products, but in small amounts. Its sweetness is comparable with sucrose at 92% but has only 38% of the calories. From galactose, it can be produced via isomerization using the l-arabinose isomerase enzyme in an immobilized form obtained from Thermotoganeapolitana
In the past few years, several studies have been done with a primary focus on the development of immobilized enzymes for future commercial use. Though the immobilized enzyme has several advantages in food processing, there are very few successful examples of immobilized enzymes in food processing. The immobilized glucose isomerase is used in the production of HFCS. The immobilized lipases are used in the production of diacylglycerols and transfree fats and/or oils. The main drawback of the immobilized enzyme system is its economics, which offset most of the other benefits of immobilized enzymes. Various other enzymes and their applications are at different stages of development. As well as the applications discussed , it has been suggested that enzymes may find extensive application in the production of flavourants in bread, beer, wine and other fermented foods as well as production of synthetic foods. Of course, numerous novel concepts have been attempted or are being pursued, such as: descaling of fish; modification of wort; beverage clarification; production of hydrolysate-based beverages for infants, geriatrics and invalids; enzymic determination as an index of food quality; food analyses; and removal of antinutritive factors from foods. The future of such processes and applications will depend heavily on economics and regulatory decisions. While it might be true that the implementation of immobilized-enzyme systems to date has not lived up to initial, optimistic expectations, the outlook is bright given current industry trends combined with the rapid evolution of immobilized-enzyme technology.
Compiled & Shared by- This paper is a compilation of group work provided by the Team, LITD (Livestock Institute of Training & Development)
Image-Courtesy-Google
Reference-On Request