Coccidiosis in Chicken
Megha Kaore* and Sunil Kolte
Affiliations: Megha Kaore: Assistant Professor, Department of Pathology, Nagpur Veterinary College, Nagpur
- W. Kolte: Professor and Head, Department of Parasitology, Nagpur Veterinary College, Nagpur
Introduction
Coccidiosis is an economically significant disease of commercial and backyard chicken in India. The global economic losses caused by coccidia infection reached 10.4 billion pounds every year (Blake et al., 2020) . Coccidiosis is highly prevalent in India especially in the monsoon season. The prevalence of coccidiosis in broilers may range between 5% and 70% depending on many factors, such as management, presence of vectors, Alphitobius, high temperature and humidity (Du et al., 2004). It is estimated that practically all farms are affected by this disease at some time of the year, either in the form of clinical or subclinical coccidiosis. The emerging poultry industry of India incurs a huge economic loss due to mortality, increased feed conversion ratios, reduced weight gains, drop in production, lower reproductive performance, cost of treatments, continuous Eimeria oocyst shedding, and increased susceptibility to other disease agents (Adhikari et al., 2020). coccidiosis is largely a disease of young animals because immunity quickly develops after exposure and gives protection against later disease outbreaks. But no cross immunity exists between species of Eimeria.
Etiology
Coccidiosis is primarily caused by intracellular protozoan Eimeria. Eimeria are obligate, intracellular, apicomplexan parasites belonging to Eimeridae family and genus Eimeria. In chicken 7 Eimeria species have been recognized as responsible for intestinal disease. These include Eimeria (E.) praecox, E. acervulina, E. mitis, E. brunetti, E. tenella, E. maxima, and E. necatrix. Among all species, E. maxima, E. tenella, E. acervulina, and E. necatrix have highest clinical and sub-clinical disease burden (Hinsu et al., 2018). Different species of coccidia have similar host infection specificities, but different parasitic sites and virulence. Cecum is the main infection site of E. tenella and the middle part of the small intestine is the main infection site of E. necatrix and E. maxima while duodenum is the site of E. acervulina. Concurrent infection with 2 or more species of coccidia is common. The morphology of coccidia oocysts is similar for most Eimeria species. They are ellipsoidal or circular shaped with a thick cell wall and sporocysts. Majority of Eimeria oocysts have ovoid shape. E. maxima (30.5 x 20.7μm) is the largest while E. mitis (15.6 x14.2μm) is the smallest as compared to other species of Eimeria. E. tenella, E. maxima, E. acervulina, and E. burnetti are ovoid while E. necatrix is oblong (Clark and Blake 2012).
Transmission
Ingestion of sporulated oocysts is the only natural method of transmission of coccidiosis. Once infected, chickens may shed oocysts in the feces for several days or weeks. The oocysts in feces become infective through the process of sporulation in about 2 days. Susceptible birds in the same flock may ingest the oocysts through litter‐pecking or the contamination of feed or water. No natural intermediate hosts exist for the Eimeria spp., but oocysts can be spread mechanically by a multitude of vectors, including insects, contaminated equipment, wild birds, and dust. Oocysts generally are considered resistant to environmental extremes and to disinfectants, although survival time varies with conditions (Shirley et al., 2005).
Life Cycle
Eimeria have a direct life cycle, characterized by high tissue and host specificity, involving stages of asexual and sexual multiplication, with three development phases: the formation of schizogony (agamogony/merogony), gametogony (gamete formation for sexual reproduction), and sporogony. As shown in Figure 1, transmission occurs via fecal-oral route and infection begins with the ingestion of sporulated oocysts. The oocyst, a thick‐walled zygote shed in fecal matter by the infected host. The process of sporulation begins immediately, under the optimal conditions of temperature, moisture, and oxygenation, oocysts sporulates and becomes infective within 24–72 hours. When sporulated oocysts are ingested, wall of the oocyst is crushed in the ventriculus, releasing the sporocysts. The sporozoites emerge from the sporocysts by the action of chymotrypsin and bile salts in the small intestine. The sporozoites contained within each sporocyst are released into the intestinal lumen by the process called excystation. The sporozoites invade the enterocytes, changing into trophozoites and starting a parasitic feeding period that lasts ~12–48 h. The parasitophorous vacuole is formed, the trophozoite begins to enlarge, and the parasite nucleus performs multiple asexual divisions forming the schizont or meront, which is full of merozoites. Approximately 3 days post infection, the mature schizont ruptures and releases the merozoites, which are fusiform and have an apical complex that allows them to move and infect intestinal epithelial cells to form additional schizont generations that reproduce asexually (Nabian et al., 2018). When the asexual reproduction phase is complete, the sexual reproduction stage or gametogony begins, occurring in three events. The first is gametocytogenesis, in which gametocytes are produced from merozoites. Second, during gametogenesis, haploid micro and macrogametes are differentiated from the gametocytes. Finally, macrogametocytes are fertilized by microgametocytes producing diploid zygotes, at which point sexual reproduction is completed; meiosis proceeds, inside the protective oocyst wall, followed by mitosis to produce the infectious sporozoites. The macrogametocyte grows quickly and forms a single macrogamete while the microgametocyte matures, breaks up and releases many small biflagellate microgametes. Once the oocyst is excreted from the animal in the feces, the third phase of the cycle, sporulation, takes places. If environmental conditions are adequate, the diploid oocyst initiates sporogony formation. With each species, the reproductive potential from a single ingested oocyst is fairly constant. The entire process takes 4–6 days, depending on species, although oocysts may be shed for several days after patency is reached. In some species (E. tenella, E. necatrix), the maximum tissue damage may occur when second‐generation schizonts rupture to release merozoites. Other species may have small scattered schizonts, which cause little damage, but the gametocytes may elicit a strong reaction with cellular infiltration results in thickening of mucosa of affected part of intestine (Walker et al., 2013).
Clinical Signs
Degree of infection and the clinical signs of coccidiosis are influenced by multiple factors including the species of Eimeria, the infective dose, host-parasite interactions and environmental conditions of the poultry house. The disease may be mild, resulting from the ingestion of a few sporulated oocysts, and may escape notice, or it may be severe as a result of ingestion of millions of sporulated oocysts. Infected birds present with ruffled feathers and signs of depression or drowsiness. Additionally, feed and water consumption are decreased, and the feces may be watery, whitish and occasionally bloody. This results in dehydration, impaired weight gain, and death in the absence of treatment. The tissue damage and changes in intestinal tract permeability and function may allow colonization by various harmful bacteria, such as Clostridium perfringens, leading to necrotic enteritis or salmonellosis. Immunosuppressive diseases may act in concert with coccidiosis to produce a more severe disease. Marek’s disease may interfere with development of immunity to coccidiosis and infectious bursal disease and chicken infectious anemia may exacerbate coccidiosis, placing a heavier burden on anticoccidial drugs (Lillehoj et al., 2000; Cervantes et al., 2020).
Diagnosis
Correct identification of Eimeria species is important for the diagnosis and control of the disease. Classical methods for the evaluation of Eimeria infections include macroscopic diagnosis with observation of clinical signs in infected animals, the location and appearance of gross lesions during necropsy; and microscopic diagnosis, which focuses on evaluating the size and shape of oocysts. Pathogenicity and characteristics lesions of each Eimeria is given in table 1.
| Eimeria species | Development Site | Pathogenicity | Lesions |
| E. acervulina | Duodenum, Jejunum
|
++ | Mucoid enteritis, causing loss of fluids, and poor absorption of nutrients
Whitish spots on serous surface, hemorrhage streak and plaques on mucosal surface in dead birds |
| E. praecox | Duodenum, Jejunum | + | Mild mucoid enteritis.
No lesion but mild hemorrhagic appearance on serous surface of duodenum |
| E. mitis | Ileum | + | Mild enteritis, causing loss of fluids and poor absorption of nutrients. |
| E. necatrix | Jejunum, Ileum, Cecum | +++ | Thickening of the mucosa and intestinal lumen filled with liquid, blood and the remains of tissue
Lesions in dead birds are observable as white and black sheets (salt and pepper appearance) |
| E. maxima | Jejunum, Ileum | ++ | Distended intestine (ballooning) with hemorrhagic spots, abundance of yellow‐orange mucoid discharge |
| E. brunetti
|
Cecum and Rectum | +++ | Thickening of the mucosa, coagulative necrosis with a caseous eroded surface over the entire mucosa. Coagulated blood and mucosal casts are apparent in the droppings |
| E. tenella | Cecum | +++ | Presence of prominent blood and severe hemorrhage with white red spots. Cecal cores, which are accumulations of clotted blood, tissue debris, and oocysts in birds surviving the acute stage |
Microscopic identification
Scrapings of the intestinal mucosa should be taken to evaluate the presence and shape of oocysts. Typically, the intestinal lesion score is complemented with counts of oocysts per gram (OPG) of feces or poultry litter through the McMaster technique. Recently, the Mini-FLOTAC has been developed as a new method for qualitative and quantitative diagnosis of infections by helminths and protozoans in several mammal hosts. The Mini-FLOTAC is a reliable and precise method of quantification for this species with the detection limit ranging between 100 and 500 oocysts per gram of excreta (OPG) (Bortoluzzi et al., 2018).
Molecular diagnosis
In field conditions, Eimeria infections are often caused by more than one species, with similar pathological lesions, making diagnosis difficult. Polymerase chain reaction (PCR) based on the amplification of regions of the Internal Transcribed Space 1 (ITS1) of the ribosomal DNA is now a widely used molecular technique for identification of different species of Eimeria (Tang et al., 2018).
Treatment
Once clinical signs appear, use of antibiotics and supportive care is advisable to minimize dehydration and secondary bacterial infection. Treatment for coccodiosis is with sulfa drugs or anticoccidial drugs. Amprolium is an anticoccidial drug available without a prescription and is a fast, highly effective treatment for coccidiosis. For disease outbreak recommended dose of amprolium is 60 g in 25 litres of water for 5-7 days.
Control measures for coccidiosis
The prevention and control of coccidiosis is based on the use of prophylactic anticoccidial drugs, vaccines, natural feed additives, and strict biosecurity on farms.
Control with anticoccidial agents
In spite of the challenges posed by drug resistance, anticoccidial drugs still remain the primary means of coccidiosis control worldwide. Fermentation‐derived ionophore and chemically synthetized anticoccidials remain the backbone of coccidiosis control. In market there are “mixed products” that are comprised of a combination of the two. The choice of product depends upon the type of birds, season of the year, type of program or other factors which affect exposure. In broilers the objective is to produce the maximum growth and feed efficiency with minimum of disease. In long‐lived birds like table‐egg layers and breeders kept on the floor, the objective is to protect birds against early acute infections and to provide long‐lasting immunity (Mesa-Pineda et al., 2021). Nevertheless, like the majority of antimicrobials, their repeated use in poultry production over time and the lack of new active ingredients in recent years has caused an increase in the resistance of strains of Eimeria spp. These resistances bring with them an increased risk of subclinical coccidiosis appearance, an increase in intestinal dysbiosis and bacterial enteritis such as Necrotic Enteritis and a consequent worsening of productive parameters. To overcome this, anticoccidials are now used under programs called dual (or shuttle) or straight rotation. In the first program, two or more anticoccidials, usually with different modes of action, are alternated in the different feeds supplied during the chicken life cycle; whereas in the second program, the same drug is used continuously throughout a production cycle, but is changed for an alternative drug after one or several flocks (Quiroz-Castaneda et al., 2015). List of commonly used anticoccidials is given in table 2
Table 2. Commonly used anticoccidials in chicken
| Compound | Category | Anticoccidial agent | Recommended dose (ppm)-Broiler |
| Ionophores | Monovalent | Monensin | 100–120 |
| Narasin | 60–80 | ||
| Salinomycin | 44–66 | ||
| Monocyclic glycosidic | Maduramicin | 5–6 | |
| Semduramicin | 25 | ||
| Divalent | Lasalocid | 75–125 | |
| Chemicals | Amprolium | 125–250 | |
| Aprinocid | 60 | ||
| Clopidol | 125 | ||
| Decoquinate | 30 | ||
| Diclazuril | 1 | ||
| Dinitolmide (zoalene) | 125 | ||
| Halofuginone | 3 | ||
| Nequinate (methyl benzoquate) | 20 | ||
| Nicarbazin | 125 | ||
| Robenidine | 33 | ||
| Mixed | Synthetic with ionophore | Salinomycin/nicarbazin | 50 |
| Narasin/nicarbazin | 54–90 (combination) | ||
| Maduramicin/nicarbazin | 3.75–40 | ||
| Semduramicin/nicarbazin | 15–40 | ||
| Monensin/nicarbazin | 40 | ||
| Synthetic with synthetic | Meticlorpindol/methylbenzoquate | 110 |
Control of coccidiosis in chicken by vaccination
The primary strategy for preventing and controlling avian coccidiosis has become the vaccination with either live or attenuated vaccines following the implementation of antibiotic bans throughout the world. The efficacy of the vaccine is highly dependent upon the species of Eimeria contained within the vaccine. These vaccines normally contain 3 or more species of Eimeria, which are most important. In the case of broiler vaccines, these are E. acervulina, E. maxima, and E. tenella. Due to the geographical diversity of Eimeria isolates, vaccine efficacy can differ significantly.
A number of new vaccines have emerged recently, including recombinant vaccines, DNA vaccines and genetically engineered live vaccines. Live vaccines use the oral introduction of low doses of Eimeria oocysts to stimulate humoral and cellular responses from the host immune system. There are several virulent live oocyst vaccines for avian coccidiosis including CocciVac®-B, CocciVac®-D, VACM®, ADVENT®, Inovocox®, Inovocox®-EM1, Immucox C1 Immucox C2 and Nobilis COX-ATM. Attenuated vaccine is used extensively primarily with layer and breeder stock owing to their relative cost and limited productive capacity. Paracox-5 and Paracox-8 are the first two early-maturing live avian coccidiosis vaccines since then Livacox D®, Livacox T®, Eimeriavax 4M, Inmuner Gel-Coc, Hipracox Broilers and other early-maturing live attenuated vaccines have been developed.
To address the emergence of drug-resistant strains of Eimeria, recombinant vaccines can be used as an alternative strategy for controlling coccidiosis. These recombinant proteins can stimulate the chicken body to produce partial immunity to one or more Eimeria spp. However, as the recombinant antigen is unable to trigger a wide range of protective immune responses like the live or attenuated parasite vaccine, there are some limitations in using a single recombinant Eimeria protein to prevent coccidiosis.
DNA vaccines can significantly reduce the shedding of fecal oocysts. DNA vaccines are less expensive compared to the recombinant vaccines. However, DNA vaccines may integrate into the host chromosome and even induce a long-lasting strong cellular immune response, affecting host cell growth.
Natural products for Coccidiosis Control
To reduce drugs in the food chain led to search for alternative strategy to the control of coccidiosis. Among the alternative measures explored is the use of natural products which include probiotics, prebiotics, essential oils and organic acids. Phytochemical compounds can be used as preventive, therapeutic, or immuno-modulators against coccidiosis.
Probiotics are usually used as live microbial feed supplements, which are ingested by chickens which can protect the integrity of the intestinal epithelial barrier and reduce oocyst shedding after being infected by Eimeria spp. Lactobacillus plantarum, Pediococcus pentosaceus, Saccharomyces cerevisiae, Bifidobacterium, Enterococcus faecium, B. subtilis, Clostridium butyricum either alone or together with other supplements may be used. In addition, the combined application of probiotics and avian coccidiosis vaccine help to improve the protective effect of the vaccine and enhance the production performance parameters of broilers.
Prebiotics are metabolites produced by probiotics that selectively stimulate the growth of beneficial bacteria in the gut and increase the number of beneficial microbiotas. Arabinoxylooligosaccharides (AOS), fructooligosaccharides (FOS), isomaltooligosaccharides (IMOS), mannan-oligosaccharides (MOS), soy oligosaccharides (SOS), xylooligosaccharides (XOS), pyrodextrins, and inulin are used in poultry farming. These prebiotics shed oocyst in feces and reduces the severity of lesions in chicken. Therefore, the use of prebiotics is emerging as a new approach to control coccidiosis.
Plant-derived adjuvants mainly include essential oils (EO) and organic acids has shown an inhibition toward the various stages of Eimeria. Some of the common EO like oregano, carvacrol, thymol, cinnamaldehyde and citral and organic acids like short-chain fatty acids (SCFAs), Glycerol monolaurate (GML), Butyric acid glycerides (BAG, Lactobutyrin) and clopidol have shown good antiprotozoal activity against Eimeria spp.
Conclusions
For the control and prevention of poultry coccidiosis proper hygienic and bio security measures should be implemented, and prophylactic anticoccidial drugs should be provided with appropriate time and recommended dose. Information gained from molecular assays can guide the poultry farmers in managing disease by allowing informed decisions on which anticoccidial compounds (traditional or naturals) or live oocyst vaccines should be used in the field. Phytochemical compounds can be used as preventive, therapeutic, or immuno-modulators against coccidiosis.
Figures
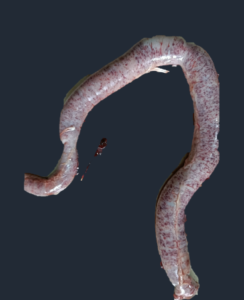 |
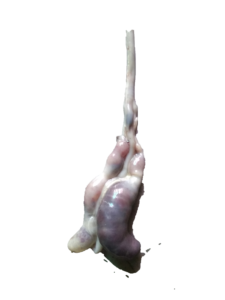 |
| Jejunum showing severe petechial hemorrhages | Distension of ceca with bloody contents and petechial hemorrhages |
 |
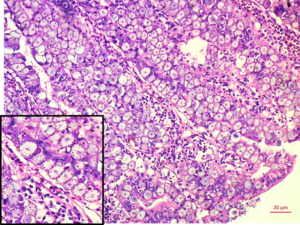 |
| Scrapping of intestinal mucosa showing oocyst (arrow) of Eimeria spp. from chicken | Duodenum: Mucosal epithelium replaced by dense sheet of macrogamete/zygotes with characteristic peripheral eosinophilic granules (inset) H&E 20x |
References:
Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, Ayoade S, Gilbert W, Adebambo AO, Jatau ID and Raman M (2020) Re-calculating the cost of coccidiosis in chickens. In: Veterinary Research, 51:1-14
Du A and Hu S (2004) Effects of herbal complex against Eimeria tenella infection in chickens. Journal of Veterinary Medicine, 51(4):194–197
Adhikari P, Kiess A, Adhikari R and Jha R (2020) An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. In: Journal of Applied Poultry Research, 29(2):515-534
Hinsu AT, Thakkar JR, Koringa PG, Vrba V, Jakhesara SJ, Psifidi A, Guitian J, Tomley FM, Rank DN, Raman M and Joshi CG (2018) Illumina next generation sequencing for the analysis of Eimeria populations in commercial broilers and indigenous chickens. In: Frontiers in Veterinary Science, 5:176–184.
Clark EL and Blake DP (2012) Genetic mapping and coccidial parasites: Past achievements and prospects. Journal of Biosciences, 37: 879-886.
Shirley MW, Smith AL and Tomley FM (2005) The biology of avian Eimeria with an emphasis on their control by vaccination In: Advances in Parasitology, 60:285–330
Nabian S, Arabkhazaeli F, Seifouri P and Farahani A (2018) Morphometric analysis of the intestine in experimental coccidiosis in broilers treated with anticoccidial drugs. In: Iranian Journal of Parasitology, 13(3):493–499
Walker RA, Ferguson DJ, Miller CM, Smith NC (2013) Sex and Eimeria: a molecular perspective. In: Parasitology, 140(14):1701–1717 doi: 10.1017/S0031182013000838
Lillehoj HS and Lillehoj EP (2000) Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. In: Avian Diseases, 44 (2):408–425 10.2307/1592556
Cervantes HM, McDougald LR and Jenkins MC (2020) Coccidiosis, In: Diseases of Poultry, Fourteenth Edition. Editor-in-chief David E. Swayne: John Wiley & Sons, Inc. (Vol. II), (pp.1193–1217)
Bortoluzzi C, Rothrock MJ, Vieira BS, Mallo JJ, Puyalto M, Hofacre C and Applegate TJ (2018) Supplementation of protected sodium butyrate alone or in combination with essential oils modulated the cecal microbiota of broiler chickens challenged with coccidia and Clostridium perfringens In: Frontiers in Sustainable Food Systems, 2:72-77
Tang X, Huang G, Liu X, El-Ashram S, Tao G, Lu C, Suo J and Suo X (2018) An optimized DNA extraction method for molecular identification of coccidian species. In: Parasitology research, 117:655–664 doi : 10.1007/s00436-017-5683-8
Mesa-Pineda C, Navarro-Ruiz JL, Lopez-Osorio S, Chaparro-Gutierrez JJ and Gomez-Osorio LM (2021) Chicken coccidiosis: from the parasite lifecycle to control of the disease In: Frontiers in Veterinary Science, 8:787653
Quiroz-Castaneda RE and Dantan-Gonzalez E (2015) Control of avian coccidiosis: future and present natural alternatives. BioMed research international, 430610, https://doi.org/10.1155/2015/430610



