CONCEPT OF VETERINARY DRUG DOSAGE & ITS CALCULATION
Veterinary Pharmaceutical manufacturers provide safe products that will effectively treat a health problem, provided they are stored and used according to label directions. Prior to licensing a product, research is conducted to determine the best injection site, route and dosage for the treatment of a particular condition in a particular species and class of animal.In case of veterinary field, vets has to treat several animals from farm to wildlife.The available medicine in the market are same for large and small animals, only difference is these medicines’s dosage are given to the said animal as per the body weight of the animal or body surface area.Different forms of medicines are there as per their need.Here in this article I have tried to give a brief idea about the different forms of medicines and its dosages calculation as per the body weight of the animals. One must follow the manufacturers guidelines regarding dosages.
DRUG DOSAGE FORMS IN VETERINARY MEDICINE
The dose is the amount of drug taken at any one time. It can be expressed as the weight of drug (e.g. 250 mg), volume of drug solution (e.g. 10 mL, 2 drops), the number of dosage forms (e.g. 1 capsule, 1
suppository) or some other quantity (e.g. 2 puffs).
The dosage regimen is the frequency at which the drug doses are given. Examples include 2.5 mL twice a day, one tablet three times a day, one injection every four weeks.
The total daily dose is calculated from the dose and the number of times per day the dose is taken.
The dosage form is the physical form of a dose of drug. Common dosage forms include tablets, capsules, creams, ointments, aerosols, and patches. Each dosage form may also have a number of specialized forms such as extended-release, buccal, dispersible, and chewable tablets. The strength is the amount of drug in the dosage form or a unit of the dosage form (e.g. 500 mg capsule, 250 mg/5 mL suspension).
The route of administration is the way the dosage form is given. Common routes of administration include oral, rectal, inhalation, nasal and topical.
The optimal dosage is the dosage that gives the desired effect with minimum side effects.
There are many factors taken into consideration when deciding a dose of drug – including animal species, age, health state, etc. Other medicines may also affect the drug dose.
Dosage instructions are written on the vet’s prescription and on the pharmacy label of a prescribed
medicine. Dosage instructions are also found on the packaging and inserts of over-the-counter medicines.
Please note that animal welfare regulations prohibit the use of expired drugs on research animals, except
if the animal is anesthetized for a terminal study. The anaesthesia drugs may not be expired under any
circumstances.
Species Code:
Can = canine
Fel = feline
Bov = bovine
Ov = ovine
Cap = caprine
Por = porcine
Rod = rodents (individual species listed by name) Rab = rabbit
NHP = nonhuman primate Av = avian
Rep = reptiles (species listed by name)
Am = amphibians (species listed by name) Fish (species listed by name)
ORAL DRUG DOSAGE FORMS
Oral dosage forms comprise liquids (solutions, suspensions, and emulsions), semi-solids (pastes), and solids (tablets, capsules, powders, granules, premixes, and medicated blocks).
A solution is a mixture of 2 or more components that form a single phase that is homogeneous down to the
molecular level. Solutions offer several advantages over other dosage forms. Compared with solid dosage
forms, solutions are absorbed faster and generally cause less irritation of the GI mucosa. The disadvantages of solutions include susceptibility to microbial contamination and the hydrolysis in aqueous solution of susceptible active ingredients. In addition, the taste of some drugs is more unpleasant when in solution Oral solutions
provide a convenient means of drug administration to neonates and young animals.
A suspension is a coarse dispersion of insoluble drug particles, generally with a diameter exceeding 1 µm, in a liquid (usually aqueous) medium. Suspensions are useful for administering insoluble or poorly soluble drugs or in situations when the presence of a finely divided form of the material in the GI tract is required.
An emulsion is a system consisting of 2 immiscible liquid phases, one of which is dispersed throughout the other in the form of fine droplets; droplet diameter generally ranges from 0.1-100 µm. Creaming, as occurs with milk, also occurs with pharmaceutical emulsions. However, it is not a serious problem because a uniform dispersion returns upon shaking.
A paste is a 2-component semi-solid in which drug is dispersed as a powder in an aqueous or fatty base. The particle size of the active ingredient in pastes can be as large as 100 µm. The vehicle containing the drug may be water. Pastes are a popular dosage form for treating cats and horses, and can be easily and safely
administered by owners.
A tablet consists of one or more active ingredients and numerous excipients and may be a conventional tablet
that is swallowed whole, a chewable tablet, or a modified-release tablet (more commonly referred to as a
modified-release bolus due to its large unit size). Conventional and chewable tablets are used to administer
drugs to dogs and cats, whereas modified-release boluses are administered to cattle, sheep, and goats.
A capsule is an oral dosage form usually made from gelatin and filled with an active ingredient and excipients. Two common capsule types are available: hard gelatin capsules for solid-fill formulations, and soft gelatin
capsules for liquid-fill or semi-solid-fill formulations.
A powder is a formulation in which a drug powder is mixed with other powdered excipients to produce a final product for oral administration.
A granule is a dosage form consisting of powder particles that have been aggregated to form a larger mass, usually 2-4 mm in diameter.
A premix is a solid dosage form in which an active ingredient, such as a coccidiostat, production enhancer, or
nutritional supplement, is formulated with excipients. They are administered to poultry, pigs, and ruminants.
A medicated block is a compressed feed material that contains an active ingredient, such as a drug,
anthelmintic, surfactant (for bloat prevention), or a nutritional supplement, and is commonly packaged in a cardboard box. Ruminants typically have free access to the medicated block over several days, and variable consumption may be problematic
PARENTERAL DOSAGE FORMS
Parenteral dosage forms and delivery systems include injectables (ie, solutions, suspensions, emulsions, and dry powders for reconstitution), intra-mammary infusions, intra-vaginal delivery systems, and implants.
A solution for injection is a mixture of 2 or more components that form a single phase that is homogeneous down to the molecular level. “Water for injection” is the most widely used solvent for parenteral formulations.
A suspension for injection consists of insoluble solid particles dispersed in a liquid medium, with the solid
particles accounting for 0.5-30% of the suspension. The vehicle may be aqueous, oil, or both. Injectable suspensions are commonly used.
An emulsion for injection is a heterogeneous dispersion of one immiscible liquid in another; it relies on an emulsifying agent for stability. Parenteral emulsions are rare because it is seldom necessary to achieve an emulsion for drug administration.
A dry powder for parenteral administration is reconstituted as a solution or as a suspension immediately prior to
injection. The principal advantage of this dosage form is that it overcomes the problem of instability in solution.
Mastitis intra-mammary infusion products are available for lactating and non-lactating (dry) cows. Lactating cow intra-mammary infusions should demonstrate fast and even distribution of the drug and a low degree of binding to udder tissue. These properties result in lower concentrations of drug residues in the milk.
Intra-vaginal delivery systems include controlled internal drug release (CIDR) devices, progesterone-releasing intra-vaginal devices (PRID), and vaginal sponges. These systems are used for oestrus synchronization in sheep, goats, and cattle.
The majority of implants used in veterinary medicine are compressed tablets or dispersed matrix systems in which the drug is uniformly dispersed within a non-degradable polymer.
TOPICAL DOSAGE FORMS
The topical dosage forms available for treating animals include solids (dusting powders), semisolids (creams, ointments, and pastes), and liquids (solutions, suspension concentrates, suspoemulsions, and emulsifiable concentrates). Of special interest are transdermal delivery systems that elicit clinical responses by carrying medications across the skin barrier to the bloodstream. Examples of these are transdermal gels and patches that are used in companion animals. Also of interest are dosage forms that are unique to veterinary medicine, such as spot-on, pour-on, and backliner formulations developed for the control of parasites.
A dusting powder is a finely divided insoluble powder containing ingredients such as talc, zinc oxide, or
starch.
A cream is a semisolid emulsion formulated for application to the skin or mucous membranes. Droplet diameter in topical emulsions generally ranges from 0.1-100 µm.
An ointment is a greasy, semisolid preparation that contains dissolved or dispersed drug. Ointments are indicated for chronic, dry lesions and contraindicated in exudative lesions.
A paste for topical use is a stiff preparation containing a high proportion of finely powdered solids such as
starch, zinc oxide, calcium carbonate, and talc. Pastes are less greasy than ointments because much of the
fluid hydrocarbon fraction is absorbed onto the solid particles. Pastes are indicated for ulcerated lesions.
A solution for topical use is a mixture of 2 or more components that form a single phase down to the molecular level. Topical solutions include eye drops, ear drops, and lotions.
A suspension concentrate for topical use is a mixture of insoluble, solid active ingredients, which are normally at high concentrations, in water or oil.
A suspoemulsion combines the elements of an emulsion and a suspension, allowing active ingredients with
widely varying physical properties to be formulated in a single product. Typically, a suspoemulsion contains one or more solvent-soluble active ingredients in an
Calculation of Drug Doses
This module gives examples of the calculations required in the dispensing of medicines.
- PERCENTAGE SOLUTIONS
Many drugs are supplied in a concentrated form, and you may be required to either reconstitute a powdered drug with water to give the desired concentration, or to dilute a liquid concentrate. Some injectable drugs are supplied at a given concentration with a dose rate in mg/kg. Remember that:
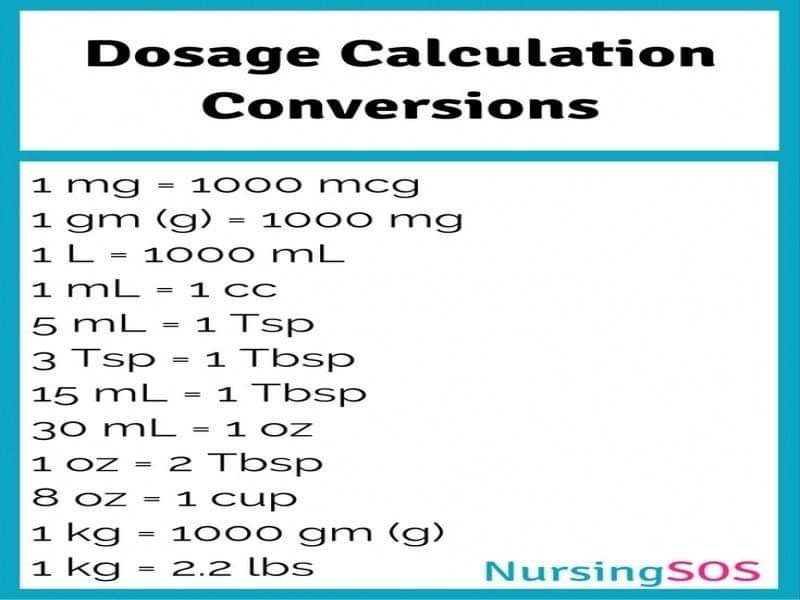
o 1kg = 1000g
o 1g = 1000mg
o 1mg = 1000mcg
o 1L = 1000mls
o To convert the concentration of a solution expressed in percentages into mg/ml simply multiply the percentage figure by 10.
o % solution = weight in grams x100 divided by the volume of solution in mls.
o Weight in grams = volume of solution in mls x % solution divided by 100.
Example – Stella (a bitch) is admitted. She weighs 20kg and requires a daily injection of a 7.5% drug in solution. The dose is 10mg/kg. Calculate the volume of the dose to be administered.
o A 7.5% solution = 75mg in 1ml.
o Dose rate = 10mg/kg.
o Dose rate x body weight (10 x 20) = 200mg.
o Mg required divided by mg in solution (200 divided by 75) = 2.67mls/day.
- ADMINISTRATION OF TABLETS
Tablets and capsules are often supplied in various strengths. They may be classified in micrograms (mcg), milligrams (mg) or grams (g). Drug doses may be calculated by either body weight (commonly) or by body surface area.
How to Calculate a Dose of Injectable Medication
Drug dosages are calculated according to body weight in kilograms and concentration of a drug (mg/mL or mg/tablet).
- Figure the dog’s body weight in kilograms. There are 2.2 pounds in 1 kg. If the dog is 32 pounds, then divide 32 by 2.2 pounds to get 14.5 kg
- If you are giving an antibiotic injection and the dose is 5 mg/kg, you will want to multiply the dose 5 mg/kg X 14.5 kg (the kg cancel out) to give you 72.5 mg total dose for the patient.
- Now, you also need to know how many mg are in 1 mL of the antibiotic solution so you can give the right dose of medication. Note that this medication gives you a percent concentration, so you will have to convert this percent into milligrams per milliliter (mg/mL). Use this formula for these types of questions: “mg/ml=%X10”. Using this formula, multiply the percentage by 10. So 2.27% X 10 = 22.7 mg/mL. Side note- (if the percent concentration was 5% it would be 50mg/mL, if it was 10% it would be 100mg/mL, and so on.)
- If the solution is 22.7 mg/mL, how many mLs will you give your patient? Take your 72.5 mg total dose needed divided by the strength of 22.7 mg/mL (mg cancel out) and you get 3.2 mLs.
You will give your patient 3.2 mLs (the equivalent of 72.5 mg) of a 22.7 mg/mL Baytril solution IV over 20 minutes. Always double check your dose before administering to the patient.
Check the bottle, the syringe, the patient’s chart, and that you have the correct patient
.
PHARMACOLOGY TERMS
Absorption Rate Constant: The rate at which a medication is absorbed from dosage site to measurement location. This is applicable to all drugs except intravenous medications.
Accumulation, Accumulation Ratio: The amount of a medication found within a bodily fluid at a specific point when a steady state has been attained. The point of equality between drug administration and drug elimination.
Accuracy: The amount of error found in the results of a scientific equation.
Activity, Intrinsic: The quality of a drug that ascertains what the biological result will be. This is also referred to as intrinsic efficacy.
Addiction: A situation where use of a drug has changed the behavior and methods of the user, creating a need for it in order to continuing using or to obtain more of it.
Affinity: The extent to which one substance tends to want to bind with another.
Allergic Response: A situation in which the body forms antibodies against a specific drug, causing a physical reaction that may or may not be severe.
Amplification: The quantity of change in determined output per unit change in input.
Analgesic: A medication that alleviates pain without the patient losing consciousness.
Anesthetic: A medication that causes loss of sensation. This is sometimes used to alleviate pain or for loss of consciousness for surgical procedures.
Antagonism: The combined result of two drugs being less than the sum of the two drugs put together. In essence, the whole is less than the sum of its parts.
Area Under the Curve: The area on a graph that falls under the curve when plotting time after administration of a drug against the plasma concentration of a drug. It is used to estimate how long it takes for a drug to be removed from the body.
AUC: The abbreviation of Area Under the Curve, or, the area of a graph that falls under the curve when plotting administration of a drug against the plasma concentration of a drug.
Availability: Also referred to as bioavailability, this is the amount of a drug dosage that is absorbed into circulation after administration of a specific dosage.
Bo: On a graph, the slope that occurs when concentration is plotted against the drug half life (or C is plotted against t).
Bioassay or Biological Assay: Establishing the strength of a chemical, physical, or biological agent, by way of a biological marker.
Bioavailability: Also referred to as availability, this is the amount of a drug that is absorbed into circulation after administration of a specific dosage.
Biopharmaceutics: The study of how the pharmaceutical expression of certain drugs can impact their pharmacodynamic and pharmacokinetic behavior.
Biotransformation: The chemical change of a drug that happens due to the effects the body has on it.
Biotranslocation: The transfer and movement of drugs in and throughout biological organisms.
Blind Experiment: A type of experiment in which the participants are unaware of the drug doses or treatments involved, so as not to affect the outcome.
Ceiling: The maximum result of a drug within a bodily tissue, regardless of the volume of the drug administered.
Chemotherapy: The treatment of cancerous or parasitic illnesses, where the drug affects only the neoplastic cells or invading organisms.
Clearance: The amount of the bodily fluid from which a drug is eliminated or excreted.
Clinical Therapeutic Index: An assessment of a drug having more safety at an acceptable level of potency or more potency at an acceptable level of safety within the recommended drug dosage.
Compartment(s): The area within the body that a drug tends to dwell in after it has been absorbed.
Compliance: The level of cooperation of a patient when following a prescribed treatment regimen.
Cross-Over Experiment: A type of experiment in which each participant receives a test preparation. The preparations are then crossed between participants in order to calculate the effects of the test preparation through various participants.
CT Index: The measure of the effects of a drug as calculated by plotting drug concentration against time.
Dependence: A physical need to maintain administration of a specific drug in order to avoid withdrawal symptoms.
Disintegration Time: The time it takes for a drug tablet to dissolve into pieces of a set size or smaller.
Dissolution Time: The time it takes for a certain amount of a drug to be reduced to a solution from a solid form.
Distribution: The volume within a person in which the administrated drug appears to have been dispersed. Also known as volume of distribution.
Dosage Form: The physical structure and appearance in which the drug to be administered is in for use.
Dose: The amount or form of a drug that is given to a user.
Dose-Effect Curve: On a graph, this is the result of plotting the dose of a drug against its effect on the bodily system.
Dose-Duration Curve: On a graph, this is the result of plotting the dose of a drug against its duration of time in the body.
Drug: A substance used in the prevention and treatment of illness or disease.
Drug Abuse: The misuse of a drug resulting in potentially destructive consequences.
Drug Dependence: Also referred to addiction, this is a situation where use of a drug has changed the behavior and methods of the user, creating a need for it in order to continuing using or to obtain more of it.
Dummy: A form of treatment that is meant to have no effect on the user, yet imitates the contrasting drug in every way. This is also known as a placebo.
Effective: A situation where an administered drug is successful in attaining its purpose.
Efficacy: The ability of a medication to produce a change in its intended cell receptor.
Elimination Rate Constant: On a graph, this is the result of plotting the logarithms of concentration against time.
Equipotent: Being equally effective or equally able to produce the drug effect of certain strength.
Equivalence: When drugs provide identical results when administered in the same amount, or those that contain equal dosages of the same type of drug, yet are named differently.
Experiment: Also called a bioassay, this is the process of establishing the strength of a chemical, physical, or biological agent, by way of a biological marker.
First Order Kinetics: The relationship of the speed of a chemical reaction in proportion to the concentrations of the reactants.
First Pass Effect: The absorption of a drug through the liver or intestines when taken in through the gastrointestinal tract but before reaching systemic circulation.
Food and Drug Administration: A federal organization responsible for ensuring compliance with the Food, Drug and Cosmetic Act.
Generic Drugs: Drugs that have exactly the same ingredients and effectiveness as another, named drug or formulary.
Habituation: A psychological feeling of need for a certain drug due to its effects on the body.
Half-Life: The time it takes for a drug concentration within the body to be reduced by one half of its original amount.
Harrison Act: A federal law regulating the distribution, transport, and manufacture of all narcotics.
Hazard: A drug that has the ability to cause bodily harm.
Hypersensitivity: The necessary condition for a person to show an allergic response to a drug.
Hypnotic: A medication that produces an effect that causes a change in consciousness or is similar to a state of sleep.
Idiosyncratic Response: An abnormal response from a drug that is specific to the person having the response.
Infusion Kinetics: The plasma concentration of a drug over a long period of time as it is proportional to the rate of the drug administration and inversely proportional to the rate of excretion and the area through which the drug is distributed.
Intrinsic Activity: The quality of a drug that ascertains what the biological result will be. This is also referred to as intrinsic efficacy.
Latent Period or Latency: The period of time between administration of a drug and the time at which an effect is achieved.
Loading dose: The first dose of a series that is larger than subsequent doses.
Maintenance Dose: The doses in a series that follow the initial loading dose.
Median Effective Dose: The dose of a drug calculated to produce a result in 50 percent of the users of whom the drug was administered.
Metameter: A term used to label the measurement of change during biological testing.
Multiple Dose Regimens: A treatment schedule for a drug in which it is given at certain intervals.
Narcotic: A drug that is able to create an analgesic effect, which may sometimes induce an altered state of consciousness.
National Formulary: A reference publication produced by the American Pharmaceutical Association that gives standards of purity for each drug.
Negative Control Drug or Negative Control Procedure: A procedure incorporated into an experiment that it should not affect the experimental system in the same way as the independent variable.
Parameter: During an experiment, one of the components that can be controlled to remain constant throughout the procedure.
Pharmacodynamics: The study of how drugs produce their effects on the body.
Pharmacogenetics: The study of the inheritance of certain interactions from drugs on the human body.
Pharmacokinetics: The study of absorption, distribution, and biotransformation of drugs on the body.
Pharmacology: The study of the features and characteristics of drugs and medications.
Placebo: A form of treatment that is meant to have no effect on the user, yet imitates the contrasting drug in every way. This is also known as a dummy.
Positive Control Drug: A drug used in an experiment that has the expectation that its results will be similar to those of the independent variable.
Potency: The strength of a drug in terms of the concentration or amount administered.
Potentiation: A situation where the result of one drug is increased by the use of another drug that has no effect.
Priming Dose: The first dose of a series that is larger than subsequent doses.
Prodrug: A substance with little action that becomes more active after being in the body.
Precision: The accuracy with which certain values of input can be understood by measured values of output.
Receptors: The part of a cell that responds to an administered drug.
Reference Standard: A drug with specific aspects that is used as the foundation of comparison with other substances that have similar aspects.
Reliability: The degree to which the drug and organism relationship is reproducible if it is studied again under similar conditions.
Risk: The probability that damage will result from exposure to a specific agent.
Selectivity: The ability of a drug to affect one type of cell over others.
Sensitivity: The ability of a specific group to respond to a drug in a certain way compared to other organisms.
Side Effects: Undesirable effects from drug treatment that are not intended as part of the therapeutic effect.
Standard Drug: Establishing the strength of a chemical, physical, or biological agent, by way of a biological marker.
Specificity: The ability of a drug to show only one type of result.
Standardized Safety Margin: The amount of a drug that is effective in almost all of the population that must be surpassed in order to produce a fatal effect on a minimum amount of a population.
Supersensitivity: An excessive amount of sensitivity to a drug.
Synergy: The use of two drugs together provides a greater effect than the sum of the original drugs.
Tachphylaxis: The building of tolerance to a drug after repeated administrations.
Therapeutic Index: A number that measures the relative safety of a drug.
Therapeutics: The discipline and actions of returning patients to a healthy state.
Threshold Dose: A dose of a drug that is just enough to produce its desired effect.
Time Concentration Curve: On a graph, the time concentration curve is the relationship between the dose of a drug and its latency period.
Tolerance: The reduced effectiveness of a drug after repeated administrations.
Toxic Effects: An effect of a drug that is harmful or lethal to the user.
Toxicology: The study of the effects of poisonous substances on the body.
United States Pharmacopoeia: A reference book that defines approved drugs and sets standards for their purity.
Validity: The amount of error found in the results of a scientific equation.
Volume of Distribution: Also known as distribution, this is the volume within a person in which the administered drug appears to have been dispersed.
Zero Order Kinetics: A condition in which the speed of an enzymatic reaction is independent of the strength of the substrate.
ED50—-minimum effective dose for 50% of the test population
LD50—-lethal dose in 50% for 50% of the test population
Therapeutic Index——Margin of Safety. (LD50 /ED50)
First Pass Effect—-Drug reaches liver through hepatic portal system, liver filters out drug, drug never reaches target
Withdrawal time——-Amount of time that must pass from administration of a drug before meat can go to market.
Half Life——Amount of time to decrease the amount of a drug in the bloodstream by 50%
Agonist——-Drug with an affinity for particular cellular receptors
Antagonist——-Drug that binds to a cellular receptor, blocking the action of the hormone or substance that uses that receptor.
Adrenergic——–Characteristic of epinephrine, or with activities like epinephrine – sympathomimetic
Cholinergic———-Characteristic of acetylcholine, parasympathomimetic
Opiate————Sedative narcotic containing opium or derivatives thereof
Opioid———–Synthetic narcotic with opiate activities
Potency————-Power of a pharmaceutical to produce its effects
Teratogenic————Pertaining to the production of physical defects in an embryo, literally monster creating
Please note – Dosage information given in this article which is being shared on social media should be used as a guideline only. Always consult your vet doctor or vet healthcare specialist before changing the dosage of any medicines.

Reference-On Request
Compiled & Shared by- Team, LITD (Livestock Institute of Training & Development)
Image-Courtesy-Google
Reference-On Request.



