CONTROL OF MG (Mycoplasma gallisepticum) IN COMMERCIAL LAYERS
Compiled, & shared by-DR. RAJESH KUMAR SINGH, (LIVESTOCK & POULTRY CONSULTANT), JAMSHEDPUR Post no 1405 Dt 19/09//2019
JHARKHAND,INDIA 9431309542, rajeshsinghvet@gmail.com
Mycoplasma gallisepticum (MG) is a main causative agent of complicated chronic respiratory disease (CCRD). MG affects also the egg production in chickens and results in reduced feed conversion efficiency, condemnation and downgrading of broilers carcasses at slaughter because of airsacculitis. Consequently, MG infection increases medication costs and decreases hatchability and quality of one day old chicks . Different types of vaccines are used to prevent MG infections in poultry. Most of these vaccines are either killed (bacterins) or mutant live ones, namely ts-11 that showed significant impact in protecting breeders and layer flocks. In spite of the availability of MG vaccines, the level of protection conferred by these vaccines to poultry flocks is not consistent.
Mycoplasmosis, commonly known as chronic respiratory disease of chickens, has existed in our country since time immemorial and was largely controllable. Recently, however, it appears to have emerged with renewed vigour, has become more common and a serious challenge to the industry.
Mycoplasma gallisepticum (MG) is a common respiratory disease in commercial layers around the world. Mycoplasma are a small primitive type of bacteria and various species of Mycoplasma are host-adapted to different animal species. Chickens can also be infected with the generally less pathogenic Mycoplasma synoviae (MS) and several other Mycoplasma species that are considered non-pathogenic. Besides infecting chickens, MG causes serious respiratory disease in turkeys and has been found in a number of other avian species. In parts of the U.S., a common wild bird, the house finch, has been found to be infected with MG. MG can be transmitted vertically from infected breeders through the hatching egg to the chicks, horizontally from bird-to-bird, from contaminated surfaces or through the air for short distances. In the middle of the 20th century, primary breeders recognized the importance of MG and their role in preventing vertical transmission. As a part of the United States Department of Agriculture (USDA) National Poultry Improvement Plan (NPIP), MG was eradicated from primary breeding lines. Since then, breeders have considered it their duty to supply MG- and MS-negative parent flocks to the worldwide layer industry. In most countries, parent flocks are maintained free of MG so that MG-negative commercial chicks can be supplied to the commercial egg producers. In many cases, the MG-negative status is not maintained on commercial layer farms. The typical multi-age rotating population of large layer farms permits flock-to-flock horizontal transmission of MG so that the infection can never be eliminated. Consequently, layer producers have had to learn to live with MG and minimize the effects with vaccination and medication programs.
Cause
The disease is caused by a mycoplasma known as Mycoplasma gallisepticum (MG).The strains of MG may differ markedly in virulence, that is, in the degree of their disease-producing power. Some strains of MG are mild while others are highly virulent (very harmful). Mycoplasma is similar to bacteria, but lacks a cell wall. Lack of wall makes MG extremely fragile. They are easily killed by disinfectants, heat, and sunlight. They remain alive in the environment, outside the bird, for only up to 3 days. For this reason, MG is fairly easy to eliminate on single-age, all-in all-out poultry farms.
Spread
M. gallisepticum enters through the respiratory tract and/or eye. It is transmitted through direct contact of infected birds, or through the environment, dust, soil, drinking water, and feed. It spreads only short distances by air (horizontal transmission). Once a chicken is infected, the infection is of long duration. The organism is present in the respiratory tissues in high levels and is shed into the environment and eggs. After several weeks, the level of infection and shedding of the organism decreases. But even then the infection persists in the flock indefinitely and the chickens may shed the organism periodically, especially following a period of stress. This makes elimination of MG extremely difficult in multi-age breeder and laying complexes. The disease is spread from farm to farm mainly by movement of contaminated people, equipment, and vehicles. Thus basic biosecurity is the best means of preventing introduction of MG into layer and breeders complexes. Because once infected, birds remain carriers for life. Egg transmission (vertical transmission) to broiler progeny occurs at a low level from infected breeders. However, horizontal infection then readily occurs in broiler houses. The organism is also spread by wild birds. Wild birds may become infected and shed MG.
THE DISEASE MG
is primarily a respiratory disease in chickens, but the most significant effects in layers are a reduction in egg production and a slightly elevated mortality rate, likely due to secondary bacterial infections with the presence of respiratory lesions. Layers can be infected by a number of viral and bacterial respiratory diseases which share many of the same lesions and symptoms, and two or more such diseases can occur simultaneously in a flock. When dealing with respiratory diseases in layers, it is important to get an accurate diagnosis so it is known exactly what diseases are present. Each disease (and combination of diseases) is unique and has its own optimum control measures and vaccination methods. Diagnosis of MG should involve a combination of observation of typical symptoms and lesions of MG in a flock, along with either a serological change or isolating the organism in correlation with the onset of symptoms. Serology testing utilizing the plate agglutination, hemagglutination-inhibition (HI), or ELISA methods will detect antibodies specific to MG. Depending on prior vaccinations given to the flock, a presence of MG antibodies or an increasing titer may indicate infection with a field stain of MG. Other laboratory methods, such as culture or PCR, are a direct indication of the presence of the MG organism.
The most noticeable symptom of MG infection in an adult layer flock is an extended drop in production (often 10-15%) occurring over a 4-6 week period. Production is slow to recover and often never does recover to the pre-infection level or a normal production level for the age of the flock. Shell quality may suffer some effects, but this symptom is not as consistent as with other respiratory diseases such as infectious bronchitis or Newcastle. Mortality may be somewhat elevated with the presence of respiratory lesions. Tracheas can be quite inflamed with some extra mucus and exudate, but do not have the firm trachea plugs typical of laryngotracheitis (ILT) or wet fowl pox. Chronic airsacculitis with cheesy cores can be found, especially in the anterior airsacs.
The Disease Process ———-
After entering the respiratory tract, the organism attaches to the lining epithelial cells. It secretes hydrogen peroxide which causes oxidative stress on the cell membrane. This facilitates its penetration. Penetration of M. gallisepticum into cells occurs within 5 minutes and the number of intracellular mycoplamas (i.e., inside the cells) increases in 24 hours. Spread to other organs, for example, brain, indicates that brief systemic (blood) infections occur. Infection to oviduct may result because of its closeness to infected abdominal air sacs. Although always considered as a pathogen (disease-producing organism) of the mucosal surface of the respiratory tract, MG is able to penetrate cells. Once inside the cells, it easily avoids the action of antibodies and some antibiotics. This, in turn, enables the organism to pass through the respiratory mucosal barrier and cause systemic infection. Thus, the cell invasion plays a major role in the systemic spread of M. gallisepticum and in escaping from the host defences. This, in turn, allows its survival in the bird and persistence of infection, hence the name chronic respiratory disease (CRD).
Incubation Period
The incubation period varies from 6-21 days. However, development of clinical signs can be highly variable depending on M. gallisepticum strain virulence (disease-producing power), complicating infections, and environmental and other stress factors. Thus, many variable factors influence the onset and extent of clinical disease. Therefore, meaningful incubation pe riods cannot be stated. Layer birds usually develop clinical infections at the onset of egg production.
Clinical Signs
The most characteristic signs are tracheal rales (abnormal respiratory sounds), nasal discharge, and coughing. Feed consumption is reduced, and the birds lose weight. In laying flocks, egg production drops. However, flocks may show serological evidence of infection without clinical signs. Male birds may show more prominent signs, and the disease is more severe during winter. In broiler flocks, most outbreaks occur after four weeks of age. Signs are usually more marked than those observed in mature birds. Severe outbreaks with high morbidity (birds affected) and mortality, seen in broilers, are usually due to concurrent infections are usually due to concurrent infections and environmental factors.
Morbidity and Mortality
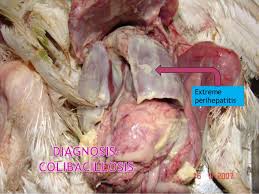
Although M. gallisepticum is the main cause of chronic respiratory disease, other organisms usually cause complications. Severe air sac infections, usually called “complicated CRD (CCRD)” or “air sac disease” is the condition more commonly observed in the field. Newcastle disease (Ranikhet disease), infectious bronchitis, or even LPAI virus may precipitate outbreaks of M. gallisepticum infection. E. coli is the most common complicating organism. The effects of M. gallisepticum, E. coli, and infectious bronchitis (IB) virus infections alone or together have been reported. As mentioned earlier, recently co-infection of M. gallisepticum and LPAI virus ((H3N8) in chickens has also been reported. Mortality may be negligible in adult laying flocks, but there can be reduction in egg production. In broilers, mortality may vary from low in uncomplicated disease to severe in complicated outbreaks. Poor growth adds to further losses
Postmortem Lesions
Lesions may not be visible or consist mainly of inflammatory exudate in nasal and paranasal passages, trachea, bronchi, and air sacs. In severe cases, there is airsacculitis and air sacs usually contain cheese-like material (caseous exudate) resulting in high mortality. These lesions are mainly because of the complication caused by E. coli infection. Oviducts distended with exudates (salpingitis) have been associated with decreased egg production in M. gallisepticum-infected flocks.
TREATMENT
In the field, many cases of M. gallisepticum infection are complicated by other diseaseproducing bacteria. Therefore, effective treatment must also attack the secondary invaders. Most strains of mycoplasma M. gallisepticum are sensitive to a number of antibiotics, but are resistant to penicillins or other antibiotics which act by inhibiting cell wall biosynthesis. M. gallisepticum may develop resistance to commonly used antibiotics. Antibiotics are usually given in feed or water. A combination of colistin, tylosin, ciprofloxacin, and bromhexidine (a bronchodilator) in drinking water at the dosage of 1ml/2 litres of water for 3-5 days has been found effective. In case of severe infections, amikacin and tylosin combination each as 15 mg/kg body weight can be given as injection. This is very helpful. However, complete elimination of M. gallisepticum from all birds in an infected flock by antibiotic treatment is an unrealistic hope. The treatment should be regarded as a method for short-term control of disease and economic losses, rather than as a long-term solution to the problem.
INACTIVATED MG BACTERINS
A bacterin is an injectable solution containing inactivated MG organisms in a water-in-oil emulsion. It may be produced as a single antigen MG product, or in a combination with Newcastle and infectious bronchitis. This type of vaccine should produce a strong antibody response and all injected birds should test strongly positive for MG antibody 2-3 weeks after the injection. The protection from bacterins seems to be best in early production as monitoring often shows the antibody response begins to decline in midcycle production (positive serology dropping below 100% after 40-50 weeks). This may allow the field strain of MG to spread through flocks in mid-late production as evidenced by a return to 100% positive serology. Hopefully, this transition is slow and mild enough that production is not affected, but in some cases, production can be negatively impacted. Routine monitoring of MG antibody titers and correlating those results with any observed production drops will allow a producer to identify if this is happening.
POX-VECTORED MG
A relatively new type of vaccine is a recombinant or vector vaccine. These are vaccine viruses, such as fowl pox or HVT Marek’s disease vaccine, that have been genetically-engineered to contain selected genes for the immunogenic proteins of a second pathogen, like MG. As the vector virus reproduces, it produces the proteins coded by the inserted genes from the second pathogen. These proteins stimulate the immune system and provide immunity against that second pathogen without any risk or stress from reacting to the live virus or bacteria as in traditional vaccines. A pox-vectored MG vaccine is used like a normal pox vaccine in the growing period. While this concept has proven to work well for some pathogens, sometimes the protection from the vectored pathogen is not as strong as from traditional live or killed products. A comparison of protection from three types of MG vaccines reported in Avian Diseases failed to demonstrate any protection from a recombinant fowl pox-MG product.
1 LIVE MG VACCINES
Historically, the first attempt to vaccinate for MG utilized a naturally-occurring mild strain known as “F-strain”. It had no detrimental effects when inoculated into growing pullets and when given prior to a more pathogenic field challenge, F-strain was able to prevent the negative effects of the disease. Originally, F-strain was grown in large liquid batches by local laboratories and applied as a fresh culture without any packaging step. That original version retained some virulence and could not be used in areas where it could spread to turkeys or susceptible adult layers. In the 1980’s the F-strain was commercialized and adapted as a live freezedried vaccine. As such, the strain has become less virulent and has been shown to have very little pathogenicity or spreading potential. One product is licensed for spray application and one for drinking water, but field experience has shown better seroconversion when the product is applied by eyedrop, probably due to a higher dose getting in the bird. It has also successfully been applied when mixed with infectious laryngotracheitis vaccine and given by eyedrop. F-strain is strong enough to remain in the bird for its life and provide a permanent competitive exclusion against infection by the field strain. Two other types of live MG vaccine are also marketed, a 6/85 strain and a TS-11 strain. A summary of the traits of the live vaccines is presented in the table below. In some cases, the protection from these two milder live vaccines appears to decline in mid-production and flocks can experience an outbreak of MG field infection. Diagnosis of such a break is based on a combination of symptoms, lesions, and increasing antibody titers as discussed earlier. When this type of late MG reaction is consistently found in flocks previously vaccinated with one of the milder vaccines, it may indicate the need for a stronger product, like F-strain, or perhaps the need to enhance the protection with additional medications or vaccinations.
MG infection can significantly affect production and profitability of commercial layer flocks. With the aid of some simple diagnostic methods and vaccination techniques, the disease can be relatively easy to diagnose and control. The world’s layer industry may not be able to totally eliminate MG anytime soon, but by utilizing these basic methods, we are able to prevent the major negative economic effects of the disease.
Prevention and Control
The continued presence and high incidence of MG in commercial poultry indicates that earlier efforts at its eradication were not very successful. Therefore, a three-prong vigorous attack is required to deal with the problem of mycoplasmosis. It is extremely important that all the three measures are applied simultaneously. This is because each one has its own advantages and limitations, as explained below. However, if applied together, they bear fruit.
1. Use of antibiotics: For example antibiotics such as kitamycin and colistin can be given in the feed regularly as antibiotic growth promoters. They are very helpful in the prevention of CRD. Limitation: The antibiotics will have no effect on the organisms present inside the cells, and therefore complete elimination of M. gallisepticum in an infected flock cannot be achieved. The organisms present inside the cells escape the action of antibiotics; birds become carriers of infection and continue to shed the organisms. This results in contamination of the environment and spread of infection.
2. Use of vaccines:
(a) Killed vaccines: M. gallisepticum killed vaccines (bacterins) protect young birds from infection with virulent M. gallisepticum and commercial egg layers from M. gallisepticum-induced drops in egg production. Limitation: Antibodies will have no effect on the organisms present within the cells. However, killed vaccines have been shown to reduce but not eliminate colonization by M. gallisepticum following infection. They are thought to be of low value in long-term control of infection on multiple age production sites. Regarding effect of killed vaccines on the spread of the organism, there is some reduction in shedding, but vaccination does not reduce horizontal spread of M. gallisepticum between laying hens.
(b) Live vaccines: These are of three types: F strain vaccine, 6/85 strain vaccine, and ts-11 vaccine . Because of their greater safety (relative avirulence and low potential for transmission to unvaccinated flocks), both 6/85 and ts-11 vaccines may be preferred than F strain when M. gallisepticum vaccination is necessary. Limitation: Because they are live vaccines, there are concerns for the safety of F, 6/85, and ts-11 strains. Even though these vaccines are generally very safe, they may have the potential for infecting unvaccinated flocks. Live vaccines should be used very carefully and administered following strictly the manufacturer’s instructions and with careful consideration for the safety of unvaccinated flocks.
3. Management: Because M. gallisepticum can be transmitted by egg, maintaining chicken flocks free of M. gallisepticum is possible only by starting with breeding stocks that are free of the infection. Then, they should be reared with adequate biosecurity to avoid introduction of the organism. To sum up, each measure has its limitations. Moreover, because of overcrowding and lapses in biosecurity, maintaining M. gallisepticum-free poultry flocks may be difficult or impossible. It is therefore of utmost importance that all the three measure s, namely, administration of antibiotics, proper vaccination, and good management must be exercised together with full force to keep mycoplasmosis under control.
Why is M. gallisepticum a serious challenge to commercial poultry?———
There are four basic reasons:
1. Our country witnessed the arrival of Avian Influenza in February 2006. Because of the stringent measures taken by the Government of India, the highly pathogenic form of the disease (HPAI) was brought under control but the low pathogenic form of the virus (LPAI) kept circulating, became endemic, and got itself firmly established as a permanent resident. Not only that, but during the six intervening years, it sharpened its razor and increased its disease-producing power (virulence). This happened because of the constant point mutations that occurred in its genes encoding haemagglutinin and/or neuraminidase surface glycoproteins (antigenic drift). Antigenic drift gradually changes the structure of haemagglutinins and neuraminidases resulting in increased disease-producing power. As a result, infections by drift variants of LPAI in the field have become widespread and very harmful. More recently, co-infection of M. gallisepticum and LPAI virus ((H3N8) in chickens has been reported. In the light of this, it is suggested that LPAI may predispose the birds to M. gallisepticum infection and vice versa. That is, infection with M. gallisepticum may predispose the bird to the variant form of LPAI. This may account for the increased occurrence and increased mortality associated with M. gallisepticum nowadays.
2. An important feature of M. gallisepticum is its antigenic variability. To put it simply, it keeps changing its surface proteins (antigens). Proteins constitute more than two-thirds of the mycoplasma membrane, the rest being membrane lipids. The plasma membrane of M. gallisepticum contains approximately 200
polypeptides which are associated with surface antigenic variation. These antigens have been identified. They play key roles in the development of the disease (pathogenesis) and immune responses. M. gallisepticum is highly committed to antigenic variation and changes in the expression of the surface proteins. This leads to the production of “atypical” or “variant” strains of M. gallisepticum. As a result, the immunity produced by the administered vaccines is unable to deal effectively with the “variant” form of M. gallisepticum, and the infection may persist despite vaccination. Thus, antigenic variation is one of the simplest strategies adopted by this organism to avoid destruction by the immune defences of the bird.
3. Another characteristic feature of M. gallisepticum its phenotypic switching. To put it simply it is a very complex way in which this organism changes its surface antigens not only while outside the birds but also while inside the bird. These changes allow the organism to avoid the immune response of the bird, and therefore it persists in the bird for a longer period. M. gallisepticum has an inherent mechanism of making rapid and sudden changes in its expression of proteins in response to antibodies. Thus, this organism uses phenotypic switching for its survival in the bird. In other words, M. gallisepticum may persist in the bird even in the presence of systemic or local antibody (carrier state).
4. Yet another important factor in the control of mycoplasmosis is the complex immune mechanisms involved in protecting the bird following vaccination. For example, there is poor correlation between the levels of circulating antibody and protection of the bird. It has been found that antibody in the respiratory secretions play a role in resistance against M. gallisepticum. Respiratory tract antibodies inhibit attachment of the organism to tracheal epithelial cells, which is one important mechanism of protection. It has now been established that although local immunity plays a main role in controlling M. gallisepticum infection, cellmediated immune system is also involved. There is significant involvement of natural killer and cytotoxic-T cell responses. To sum up, M. gallisepticum infection is a serious challenge because antigenic variation and phenotypic switching enable the organism to escape from the immune defences of the bird. This enables it to establish a chronic infection despite a strong immune response. Moreover, M. gallisepticum may hide from bird’s defences by entering into the cells. Since antibodies and antibiotics cannot penetrate the cells, this strategy helps in its survival and persistence of the infection.
NB- In M. gallisepticum infection, there is poor correlation between the levels of circulating antibody and protection. Therefore, before killed vaccines are given, the birds must be primed with a live vaccine to ensure production of local immunity in the respiratory tract. As already discussed, local immunity gives more protection than systemic immunity of killed vaccines. Killed vaccines do not produce effective local immunity. Repeating killed vaccines twice or thrice may not be helpful. This is particularly important for hatchery.
• Antibiotics should be changed periodically to avoid development of resistance.
• Stringent biosecurity is extremely important in the control of disease for two reasons. Firstly, since M. gallisepticum spreads only short distances by air, where excellent biosecurity is practised, there have been many cases when infection has not spread to neighbouring houses within a complex. Secondly, biosecurity would prevent any infection spread by the vaccinated birds.
• Good management by ensuring good ventilation, no overcrowding, and good nutrition are very helpful.
• Finally, it is strongly emphasized that M. gallisepticum must be controlled at all cost. If this is not done, apart from inflicting the damage on its own, the disease will also act as a great predisposition to E. coli, Newcastle disease, infectious bronchitis, and possibly also to drift variant of LPAI.
• To conclude, it is unlikely that MG will be eradicated from the commercial poultry industry in the coming years. However, though biosecurity programmes and effective use of vaccines, losses can be reduced.


