DETECTION OF HAEMOPROTOZOAN PARASITES IN TICK TISSUE BY STAINING METHODS
Sudha Rani.R, Assistant Professor, SRDDL, IAH&VB, KVAFSU, Bangalore-24
Abstract
On staining the tick tissues i.e salivary gland, mid gut and ovaries by giemsa (59) and methyl green pyronin stain (59), eight was positive for Theileria by giemsa stain and seven ticks were found positive for Theileria by methyl green pyronin stain whereas in the unorganised farms 400 tick tissues were stained with giemsa (200 ticks) and methyl green pyronin (200 ticks), in which 20 ticks were found positive for Theileria by geimsa stain. Whereas 31 ticks out of 200 ticks were found positive for theileriosis by methyl green pyronin stain. None of them were positive for Babesiosis by giemsa stain and methyl green pyronin.
Key words: Haemoprotzoan parasites, vectors, sheep.
Ectoparasites are very common and widely distributed in all agro-ecological zones of tropical countries like India. Among the livestock, small ruminants are the most affected by ectoparasites of veterinary and medical importance, hindering their productivity. Economic importance of ticks has long been recognized due to their ability to transmit diseases tohumans and animals. Blood sucking by largenumbers of ticks causes reduction in live weight and anemia among animals, while their bites also reduce the quality ofhides. However, major losses come by way of their ability to transmit protozoan (Theileriosis and Babesiosis), rickettsial (Anaplasmosis) and viral diseases. The precise identification of these organisms is essential for understanding their epidemiology and classification. The methods traditionally used to detect and identify these haemoprotozoan parasites include differential staining methods. The detection of haemoprotozoan parasites in the vector for assessing the infection rate in vectors will help to curtail the risk of Theileriosis and Babesiosis in small ruminants. Hence in this study the tick tissues viz., salivary gland, mid gut and ovaries were stained by methyl green pyronin and giemsa stain to detect parasite infection.
Materials and Methods:
(a) Collection of ticks: Fully engorged ticks were collected randomly from organised and unorganised sheep farms from different parts of the Karnataka.The ticks were collected in clean glass vials covered with muslin cloth and identified before dissection. About 300 ticks were collected, washed with distilled water and cleaned with an absorbent paper then it was dissected to remove the salivary glands, mid gut and ovaries and was stained with methyl green pyronin stain and geimsa stain.
(b) Dissection of ticks: Dissection of ticks and its tissues was done as per the method described by Edward et al. (2009) with minor modifications. The cleaned ticks were held in between thumb and forefingers with the dorsal side up and dissected with a sharp blade from the posterior boarder proceeding anteriorly expose the viscera. Dissected ticks in phosphate buffered saline were examined under a dissecting microscope. Paired salivary glands visible anteriorly on either side of trachea were removed carefully with a teasing needle and transferred on to the microscopic slides for further examination. The gut had brownish strands in the central area and the ovaries were visible as a bunch of grapes towards the posterior part which were separated and spread on different glass slides in phosphate buffered saline.
(c) Staining of tick tissues: The dissected tick’s salivary glands, gut and ovaries were stained Methyl green pyronin stain and Giemsa’s stain to know the intensity of haemoprtozoan parasitic infection in the ticks as described by Tahamtan et al., 2013 and by Zajac et al.,2012.
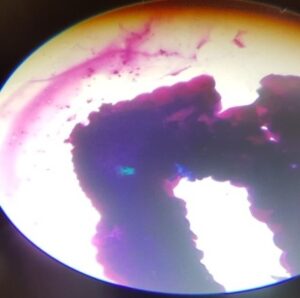
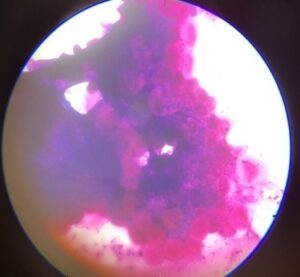
A tick is considered as positive for heamoprotozoan infection if any one of the three tissues of tick i.e salivary gland, mid gut, ovaries revealed positive parasitic stages. In infected salivary glands we can observe enlarged acini, hypertrophied with pink acinar cells cytoplasm and, even bluish green mass was also a indicative of parasite. In the mid gut cells the presence of parasitic stages was indicated by hypertrophy of infected epithelial cells and vacuolations in the cell cytoplasm. In ovaries the oocytes will show blue coloured spherical masses denoting the development stages of the parasites, but none of the ovaries showed the parasitic stages in this study.
Results:
In this study bluish green mass in salivary gland was indicative of presence of haemoprotozoan infection by methyl green pyronin staining method which was in agreement with many authors who reported the prevalence of blood parasites and detection of their vectors using methyl green pyronin and giemsa staining from abroad viz., Irvin et al.(1981); Walker et al.(1983) ; Kirvar et al.(2000) for epidemiological studies.
In geimsa staining method, the infected salivary gland will have enlarged or hypertrophied acini with enlarged sporoblast, sometimes with developmental stages of protozoa sporozoites appear in pyriform shape or dot form which can be seen in acini of salivary gland and ovaries, and midgut. In this study only 5 per cent of ticks showed dot form of parasites along with hypertrophied acini indicating positive for haemoprotozoan infection by geimsa staining method which is in agreement with Cowdry and Ham (1930) and Abdigoudarzi et al. (2013).
Under unorganised farms the tick tissues were stained with giemsa (200 ticks) and methyl green pyronin (200 ticks), in which 20 (1%) of ticks were positive found for Theileriosis by geimsa stain. Whereas 31 (15.5%) ticks out of 200 ticks were found positive for Theileriosis by methyl green pyronin stain. None of them were positive for Babesiosis by giemsa and methyl green pyronin stain. In organised farms 22 per cent of the ticks were positive for theileriosis by methyl green pyronin stain and 5 per cent of the ticks by geimsa stain. Many authors have reported the staining of cattle ticks to know the vector potentiality of ticks in disease transmission by methyl green pyronin viz., Haque et al. (2010), and Tiwari et al. (2015) whereas geimsa staining was done according to Cowdry and Ham (1930) and Zajac et al. (2012).
Fig1: Infected tick salivary Fig 2: Infected tick salivary gland : gland: Bluish green mass Hypertrophied acini & dot shaped parsaites
However, in India only limited work has been carried out in some parts of the country which was in agreement to findings of this study viz., Haryana (Sangwan et al.1988), Tripura (Das and Sharma 1991), Uttar Pradesh (Srivastava and Khan 1997; Das and Ray 2003) and Punjab (Haque et al.2010) in contrary to the findings Tahaman et al.(2013) from Varamin, Iran has reported that none of the stained salivary glands had positive reaction to methyl green pyronin that was positive for presence of Theileria, infected by another ticks previously. It seems that the life cycle of Theileria was not completed in the ticks because of less feeding period.
References:
ABDIGOUDARZI,M., 2013. Detection of naturally infected vector ticks (Acari: Ixodidae) by
different species of Babesia and Theileria agents from three different enzootic parts of Iran. J Arthropod Borne Dis.,7 (2): 164–172
COWDRY, E,V., HAM, A,W.,1930.The life cycle of the parasite of East Coast Fever in ticks
transmitting the disease. Science., 72:461–462
DAS ,S,S, SHARMA, N,N., 1991.Prevalence of Theileria infection in Hyalomma anatolicum
anatolicum ticks in north districts of Tripura (India). J .Vet .Parasitol.,5:25–27
DAS, G,, RAY,D.,2003. PCR-based detection of Theileria annulata in ticks collected from
cattle of West Bengal India. J. Vet. Parasitol., 17:11–14
EDWARDS,K,T., GODDARD, J., VARELA-STOKES, AS., 2009.Examination of the internal morphology of the Ixodid tick Amblyomma maculatum koch, (Acari: Ixodidae); a “How-to” pictorial dissection guide. Midsouth .Entomol.,2: 28-39
HAQUE,M, JYOTI SINGH, N,K, RATH, S,S.,2010. Prevalence of Theileria annulata infection in Hyalomma anatolicum anatolicum in Punjab state, India. J.Parasit. Dis., 34:48–51
IRVIN,A.D., BOARER, C.D.H., DOBBELAERE, D.A.E., MOHAN, S.M., MARAKE, R., and Ocama, J.G.R., 1981.Monitoring Theileria parva infection in adult Rhipicephalus appendiculatus ticks. Parasitol.,82:137–147
KIRVAR, E., WILKIE, G., KATZER, F., and BROWN, C. G. D., 1998.T.lestoquardi maturation and quantification in Hyalomma anatolicum anatolicum ticks.Parasitol., 117: 255-263
KIRVAR, E., ILHAN, T., KATZER, F., HOOSHMAND-RAD, P., ZWEYGARTH, E., GERSTENBERG, C., PHIPPS, P., BROWN,C.G., 2000. Detection of Theileria annulata in cattle and vector ticks by PCR using the Tams1 gene sequences. Parasitol., 120:245-54
MALLESH.P., UDAYA. KUMAR., M., and G.S.S. MURTHY, 2017.Studies on the salivary glands of Argas persicus. The Pharma Innovation Journal; 6(7): 180-183
PURNELL, R,E, JOYNER,L,P., 1968.The development of Theileria parva in the salivary glands of the tick Rhipicephalus appendiculatus. Parasitology ;58:725–732
SANGWAN, A.K., CHHABRA, M.B., SAMANTRAY, S.,1988. Comparitive efficacy of some pesticides against Hyalomma anatolicum anatolicum ticks in vitro. Indian Vet J., 65:1084-1087
SRIVASTAVA, S,C., KHAN ,M,H.,1997.Infection of Theileria annulata in Hyalomma a. anatolicum Koch. J. Vet. Parasitol.,11: 207–210
TAHAMTAN, M.H., NABIAN. S.,KHODAVEISI. M., RONAGHI.H., and ADEGHIAN,A.G., 2013.Study on prevalence of blood parasites of sheep and detection of their vectors using methyl green pyronin in Varamin, Iran. European J Exp Bio., 3(5):11-15
TIWARI.A.,SINGH,N.K., SINGH,H., JYOTI.,BHAT,S.A., and RATH,S.S.,2015.Prevalence of Theileria annulata infection in Hyalomma anatolicum anatolicum collected from crossbred cattle of Ludhiana, Punjab. J Parasit Dis .,39(1):57–61
WALKER,A.R., LATIF,A.A., MORZARIA, S.P., JONGEJAN,F.1983.Natural infection rates of Hyalomma anatolicum anatolicum with Theileria in Sudan., Res Vet Sci.,35:87–90
ZAJAC, A. & CONBOY, G. A., 2012. Veterinary clinical parasitology (8th Ed). Chichester, West Sussex, UK: Wiley-Blackwell.