DEVELOPMENT OF ACELLULAR MATRIX FROM BOVINE OMASUM USING DETERGENTS AND ENZYMES FOR WOUND HEALING IN ANIMALS
Dr. Priya Singh
Assistant Professor
Division of Surgery
College of veterinary and animal science
Rewa, NDVSU, (M.P.)
INTRODUCTION
Wound healing is a fundamental response to tissue injury that ultimately results in restoration of tissue integrity. This response is achieved mainly by the synthesis of the connective tissue matrix (Pandarinathan et al.,1998). Most wounds can heal naturally, but full-thickness skin wounds greater than 1 cm in diameter in human being needs a skin graft to prevent scar formation, morbidity and cosmetic deformities (Shevchenko et al., 2010).
The tissue engineering approach for skin substitutes has relied upon the creation of three-dimensional scaffolds as extracellular matrix (ECM) analog to guide cell adhesion, growth, and differentiation to form skin- functional and structural tissue (Zhong et al., 2010). The three-dimensional scaffolds can not only cover wound and provide a physical barrier against external infection as wound dressing, but also can provide support both for dermal fibroblasts and the overlying keratinocytes for skin tissue engineering. A successful tissue scaffold should exhibit appropriate physical and mechanical characteristics and provide an appropriate surface chemistry and nano and microstructures to facilitate cellular attachment, proliferation, and differentiation.
The use of acellular scaffolds of xenogenic origin, ECM resulting after removal of cells, as skin substitute is an acceptable modality for treating dermal wounds. Biological scaffold derived from decellularized tissues are in use as surgical implants and scaffolds for regenerative medicine because extracellular matrix secreted from resident cells of each tissue and organ can provide favourable micro-environment that affects cell migration, proliferation and differentiation (Choi et al., 2009; Zhang et al., 2009).
“Forestomach matrix” (FM) provides a number of advantages over other scaffolds and is useful in a variety of clinical and therapeutic applications, including wound repair and tissue regeneration (Lun et al., 2010). Bovine omasum matrix is selected for this study as histology shows that the lamina propria is unusually dense, whereas the abluminal side of the FM scaffold is structured as an open omasum matrix. The dense layer of ECM from the lamina propria contributed to the increased thickness and strength of FM scaffolds compared to those derived from other organs. This structure makes the FM well suited for encouraging epithelial regeneration on the dense luminal side of the matrix, and fibroblast invasion on the less dense abluminal side of the matrix, when used as a medical device for tissue regeneration. These differences serve an important role in epithelial regeneration, as the dense side acts as a barrier to cell migration, while the less dense side does not present a barrier and therefore allows cell invasion. The forestomach matrix scaffolds can be used to promote, stimulate, or increases proliferation of cells near by the scaffold attachment site as well as increases vascularization of a tissue or organ (Irvine et al., 2011). The relative size and thickness of ECM in tissue offers a solution to generate relatively large format ECM based biomaterial with good performance characteristics (Ward et al., 2014).
MATERIALS AND METHODS
The present study was conducted in the Biomaterials and Bioengineering Laboratory, Division of Surgery, Indian Veterinary Research Institute, Izzatnagar 243122 (UP). Standard reagents were obtained from Sigma-Aldrich (St. Louis, Missouri, USA) unless otherwise noted.
Preparation of acellular matrix from bovine omasum
Omasum of buffalo was procured from the local abattoir. Immediately, after collection the omasum was be kept in cold physiological saline solution containing 0.02% EDTA and antibiotic (Amikacin @ 1 mg/ml). The tissue was thoroughly rinsed with normal saline before the start of protocol. The maximum time period between retrieval and the initiation of protocol was less than 4 h. Tissue was cut from sides to obtain a flat sheet and was cut into 4 x 4 cm2 size pieces and kept in PBS for 4 h at 4oC.
Protocols for de-epithelialization and delamination of bovine reticulum
After thorough cleaning of the bovine omasum, it was kept in hypertonic solution 2M NaCl for de-epithelialization. The keratinised epithelium of omasum was easily scrubbed off by blunt surface of BP-handle. The serosal layer was separated mechanically with forceps. Microscopically, native bovine omasum showed keratinised epithelium on mucosal surface. Lamina propria is the luminal portion of the propria-submucosa, which includes a dense layer of extracellular matrix and serosal layer.
Protocols for decellularization of bovine omasum after de-epithelialization and delamination
The omasum was cut into 4 x 4 cm2 size pieces and placed in biological detergent and enzymes to carry out decellurization protocols. They were subjected to microscopic examination at 12, 24, 48, and 72 hours intervals to optimize the decellularization protocols. The prepared acellular omasum matrix was stored in PBS solution containing 1mg/ml amikacin at 4°C until use. Ionic detergent (SDS) (Rieder et al., 2004) and enzyme (Trypsin) (Gamba et al., 2002) was used in 0.5% concentration for decellularization of bovine omasum. Treatment with chemical was done upto 72 h and tissues were subjected to continuous agitation at 370C on orbital shaker at the speed of 200 rpm. The solutions were changed at every 12h intervals. Finally the tissues were thoroughly rinsed thrice (2 h each) with sterile PBS on orbital shaker and samples were collected at 12, 24, 48 and 72 h time intervals for evaluation of parameters. Macroscopic and microscopic examination was done at different time intervals.
Assessment of decellularization was done on the basis of following parameters.
The parameters for biological evaluation included:
Histopathological evaluation
The tissue samples collected at different time intervals during standardization of decellularization protocols were subjected for histological examination. The samples were fixed in 10% formal saline solution, dehydrated in ethanol, cleared in xylene and embedded in paraffin to get 5micron thin sections. The sections were stained with hematoxylin and eosin staining. Masson’s trichrome staining was done for assessing collagen fiber arrangement.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
The expression of protein bands was observed by SDS-PAGE. It was carried out in a vertical mini gel electrophoresis apparatus at a constant current of 150 V and 150 Ma, until the tracking dye reached the bottom of the gel.
DNA quantification
The DNA contents analysis before the start of protocols and after completion of protocols was done as per method described by Gilbert et al (2009).
The objective of the study was to access the effectiveness of decellularization protocols and to determine an appropriate application time for the treatments. The native bovine omasum was subjected to treatment with hypertonic solution 2M NaCl for 6 h. At 6h time interval the keratinised mucosal layer was easily scrubbed off and serosal layer was separated with slight mechanical assistance. The isolated delaminated bovine omasum was kept in 70% ethanol for sterilization for 4 h and later on washed thoroughly with distilled water for 24-48 h. The goal of decellularization was to efficiently remove all cellular and nuclear materials while minimizing any adverse effect on the composition, biological activity, and mechanical integrity of the native extra cellular matrix (Gilbert et al., 2006). Decellularization can be brought by physical, chemical, and enzymatic methods which leave a material composed of extra cellular matrix (ECM) components. In the present study chemical methods were used to prepare acellular matrix. These acellular matrices retained their natural mechanical properties and promote remodeling by neovascularization and recellularization by the host (Schmidt and Baier, 2000).
The acellular tissue matrices are biocompatible, slowly degraded upon implantation and are replaced and remodelled by the extracellular matrix proteins synthesized and secreted by in growing host cells, which reduce the inflammatory response (Pariente et al., 2001). The acellular matrices support the regeneration of tissues with no evidence of immunogenic reaction (Yoo et al., 1998). However, even after the removal of cells and cell debris the intact extracellular matrix of the acellular tissue may itself elicit an immune response (Coito and Kupiec, 1996).
In the present study two different protocols were used to obtain the acellular ECM from the delaminated bovine omasum. The delaminated bovine omasum was subjected to ionic (SDS, 0.5%) and enzyme (Trypsin 0.5%) treatment. The time of reaction (12, 24, 48 and 72 h) was optimised to obtain the acellular rECM. The delaminated bovine omasum was subjected to SDS (0.5%) treatment for 24 h became 90% acellular with mildly thick collagen fibers. The cellular debris was seen in between the void spaces of collagen fibers in the samples. Therefore, time intervals were increased to remove this cellular debris. At 48 h, complete acellularity with no cellular debris was observed. No nuclear bodies were seen and the tissue was primarily composed of extracellular matrix. Further increase in time interval to 72 h resulted in distributed and damaged collagen fibers. In the present study desired results were achieved after 48 h of treatment with 0.5% SDS detergent. The propria-submucosa layer was completely acellular. The collagen fibers were thick and arranged in longitudinal and transverse manner as compared to the native tissue. SDS is very effective for removal of cellular components from tissue. Compared to other detergents, SDS yields complete removal of nuclear remnants and cytoplasmic proteins, such as vimentin (Woods and Gratzer, 2005). Ionic detergents are effective for solubilizing both cytoplasmic and nuclear cellular membranes, but tend to denature proteins by disrupting protein–protein interactions (Seddon et al., 2004). In general, ionic detergents are used extensively in decellularization protocols due to their mild effects on tissue structure.The cell extraction was effectively achieved without significant disturbances in extracellular matrix morphology and strength. Enzymatic decellularization using Trypsin cleaves peptide bonds on the C-side of Arg and Lys, but not completely decellularized the tissue.
To measure the effectiveness of decellularization method, the DNA from cells of the samples was isolated and the DNA concentration was measured using Nanodrop. This quantification was an indirect measure to confirm the effectiveness of the processes, since the
DNA is present in active nucleus of cell. The concentration of native bovine omasum DNA (P<0.01) was 82.40±1.41g/l. Treatment with SDS (0.5% concentration) showed lowest values 4.30±0.14 g/l of DNA content in bovine omasum. Significant decrease (P<0.01) in DNA contents showed the effectiveness of treatment for decellularization. Significant decrease (P<0.05) in DNA contents was also observed by Poonam (2014) while preparing acellular cholecyst matrices from bovine and porcine origin. Treatment with SDS showed effective removal of cells. Quantification of residual DNA in animal- derived biological scaffold materials is one of technical specifications for evaluating decellularization process and immunotoxicity risk (Xu et al., 2012).
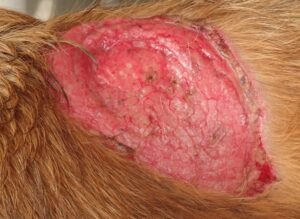
TREATMENT OF BURN WOUND USING ACELLULAR OMASAL MATRIX
BURN WOUND OVER THE DORSUM OF THE DOG
WOUND TREATED WITH ACELLULAR OMASAL MATRIX
HEALED BURN WOUND 28TH POST-OPERATIVELY
REFERENCES
Coito, A. J. and Kupiec-Weglinsky, J. W. 1996. Extracellular matrix protein by standers or
active participants in the allograft rejection cascade? Ann. Transplant. 1: 14-18.
Choi, J. S., Yang, H. J., Kim, B. S., Kim, J. Y. and Yoo, B. 2009. Human extracellular matrix (ECM) powder for Injectable cell delivery and adipose tissue engineering. J control release. 139: 2.
Gamba, P. G., Conconi, M. T., Lo Piccolo, R., Zara, G., Spinazzi, R. and Parnigotto, P. P. 2002.
Experimental abdominal wall defect repaired with acellular matrix. Pediatr. Surg.
Int.,18:327–331
Gilbert, T. W., Sellaro, T. L., and Badylak, S. F. 2006. “Decellularization of tissues and organs”.
Biomaterials, 27:3675–3683.
Gilbert, T. W., Freund, J. and Badylak, S. F. 2009. Quantification of DNA in Biologic
Scaffold Materials. J. Surg. Res., 152(1):135–139.
Irvine, S. M., Cayzer, J., Lun, S., Floden, E. W. 2011. Quantification of invitro and invivo angiogenesis stimulated by ovine forestomach matrix. Biomaterial, 32: 6351-61.
Lun, S., Irvine, S. M., Johnson, K. D., Fisher, N. J., Floden, E. W. and Negron, L.2010. A
functional extracellular matrix biomaterial derived from ovine forestomach.
Biomaterials, 31:4517-29.
Pandarinathan, C., Sajithlal, G.B., Chandrakasan, G. 1998. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol. Cell. Biochem., 181: 71-76.
Pariente, J. L., Kim, B. S. and Atala, A. 2001. In vitro biocompatibility assessment of
naturally derived and synthetic biomaterials using normal human urothelial cells. J.
Biomed. Mater. Res. 55: 33–39.
Poonam, S. 2014. Evaluation of cholecyst derived extracellular matrix for reconstruction of
full thickness skin wounds in rats. Thesis. M.V.Sc. Deemed University, Indian
Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India.
Rieder, E., Kasimir, M. T., Silberhumer, G., Seebacher, G., Wolner, E. and Simon, P. 2004.
Decellularization protocols of porcine heart valves differ importantly in efficiency of
cell removal and susceptibility of the matrix to recellularization with human vascular
cells. J. Thorac. Cardiovasc. Surg., 127:399–405.
Schmidt, C. E. and Baier, J. M. 2000. Acellular vascular tissue: natural biomaterials for tissue
repair and tissue engineering. Biomaterials, 21:2215-2231.
Seddon, A. M., Curnow, P. and Booth, P. J. 2004. Membrane proteins, lipids and detergents:
not just a soap opera. Biochim Biophys Acta., 1666:105-17.
Shevchenko, R. V., James, S. L. and James, S. E. 2010. A review of tissue-engineered skin bioconstructs available for the skin reconstruction. J. R. Soc. Interface, 7: 229.
Ward, B. R., Johnson, K. D. and May, B. C. H. 2009. Tissue scaffolds derived from forestomach
extracellular matrix. US Patent No. 12/512,835.
Woods, T. and Gratzer, P. F. 2005. Effectiveness of three extraction techniques in the
development of a decellularized bone-anterior cruciate ligament-bone graft.
Biomaterials, 26:7339-7349.
Yoo, J. J., Meng, J., Oberpenning, F. and Atala, A. 1998. Bladder augmentation using
allogenic bladder submucosa seeded with cells. Urology. 51: 221–225.
Zhang, X., Deng, Z., Wang, H., Guo, W. and Li, Y. 2009. Expansion and delivery of human fibroblasts on micronized acellular dermal matrix for skin regeneration. Biomaterials, 30: 26-66.
Zhong, S.P., Zhang, Y.Z. and Lim, C.T. 2010. Tissue scaffolds for skin wound healing and
dermal reconstruction. Nanomed. and Nanobiotech, 2: 510-525.