| Embryo Transfer Technology (ETT) in Dairy Cattle |
Compiled & Edited by Dr. Rakesh Singh,TVO, Motihari
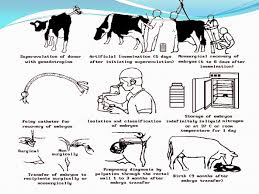
Embryo transfer is a technique by which embryos are collected from a donor female and are transferred to recipient females, which serve as surrogate mothers for the remainder of pregnancy. Embryo transfer techniques have been applied to nearly every species of domestic animal and to many species of wildlife and exotic animals, including humans and non-human primates. Within the past decade the degree of sophistication of embryo transfer procedures has evolved to permit complete utilization of non-surgical procedures in the cow, long term embryo culture and storage with cryopreservation, and, more recently, micromanipulation and many techniques associated with genetic engineering. This is a rapidly developing science with a very short lag-time between discovery and application. The potential applications continue to increase with the development of new technology. It is also called ovum transfer. It is the collection of fertilized ovum from the donor prior to its attachment/nidation with uterus and transfer into surrogative/recipient mother for completion of gestation period. The cow from which we get the embryo is called as donor. The cow to which we transfer embryo is called recipient. In AI, semen is inseminated in different cows; the genetic potential of male is distributed which is same for all cows but different cows have different potential. In embryo transfer, genetic potential of female is distributed. Every cow produces one egg at the time of estrus. Donor cows are induced to produce more than one egg at the time of estrus. These eggs are inseminated and then these fertilized eggs/embryos are taken from donor. At the same time recipient cows, having low genetic potential, are also prepared and one embryo is placed in each cow. In this way more than one calf can be obtained from one donor.
Advantages of Embryo Transfer: · It improves the genetic potential · It increases the productivity · It increases the economic benefit · It increases the disease resistance · It increases the no. of calves in life time · It reduces the generation interval · It increases the selection intensity · Import and export is easier because it is done in the form of embryo in which transportation is easier, genetics is diversified, and cost is decreased.
· Selection of donor · Selection of recipient · Synchronization of donor and recipient · Superovulation of donor · Insemination of donor · Collection of embryos · Evaluation of embryos · Transfer or storage of embryos.
· It should be from known fertile blood line i.e. we must have pedigree record · It should have calved once earlier so heifer as a donor is not required. · Donor should have known calving history or easy calving history. · It should be a regular cyclic animal. · It should be a disease free animal e.g. T.B, brucellosis · It should have been vaccinated · It should be high producing and reproduction wise normal
Production of more than one eggs by the use of hormones or drugs is called super ovulation. PMSG and FSH are the hormones used for super ovulation. FSH is short acting and PMSG is long acting. Repeated FSH injections have to be given, while PMGS is given at once. At day 10 of estrous cycle CL is present. So give 5 mg of FSH at morning and 5 mg at evening. Similarly FSH is given 5 mg morning and 5 mg evening on day 11, 5 mg morning and 5 mg evening along with PGF2α on day 12, and 5 mg morning and 5 mg evening on day 13. In this way FSH is given for four days two times a day intramuscularly. On day 14, donor will be in heat and there will be ovulation of more than one eggs.
The reproductive cycle of donor and recipient should be at the same level. We have 100 cows which are non pregnant and we have to bring them in heat in a small given period. Synchronization means to bring the events together. We synchronize all these cows. Out of 100, on palpating 20 are already in estrus, 20 are in proestrus that may come in heat after two days, 15 are in post estrus they are just in ovulation, and 30 are in diestrus. Other 10-15 are in anestrous. The function of PGF2α is to regress the CL. This purpose can be used anywhere. d1 d6 d11 d14 d17 Estrus: ∙———-∙———-∙——∙——∙ CL PGF2α Heat
d1 d3 d9 d11 d14 d17 Proestrus: ∙—-∙————∙—-∙——∙——∙ Heat CL PGF2α Heat
d1 d4 d11 d14 d15 Metestrus: ∙——∙————–∙——∙—∙ CL PGF2α Heat
d1 d3 d9 d11 d14 d20 Diestrus: ∙—-∙————∙—-∙——∙————∙ PGF2α Heat CL PGF2α Heat · Animal in estrus do not have any CL. 6 days later there will be CL. This CL will persist for 11-17 days. · Proestrous animal comes in estrus after one or two days. They do not have CL. So no response of PGF2α on day third. On day 9, CL will present which remain for day 20. · Metestrus animal was in estrus two days earlier. On day four CL will be formed which stays in ovary till day 15. · In diestrus animal has functional CL. Give PG on first day, on day 3 they come in heat. They will develop CL on ovary on day 9. 6 days will be taken by CL to develop. On day 20 CL will persist. · Anestroue do not respond to any one.
Inseminate donor in standing heat. Inseminate two or three times after twelve hours and each time go for double dose.
Until 1975, embryos were collected surgically. After anesthesia (general or local), entering the uterus and collect embryos. It was very difficult to collect from donors at farm. The cost of collection was too much. Another problem was, some damage may lead to future adhesion in reproduction tract. People tried to collect without surgery. Non surgical method is preferable now because there is no damage or very little damage that provides repeatability for the donor to donate embryos and made collection at farm level possible. Disadvantage of this method is that we can only collect the embryo when it enters the uterus. But with surgery we can collect embryos while they are in oviduct. Procedure of Collection: · Place the donor in crushes, wash the perennial region. Evacuate rectum from feces and evaluate no. of CL on both ovaries, it will tell the no. of embryos. Older cows suck air in rectum and uterus, so very old cows are not used but if they are good we can use them. Apply a bally band that would create a positive pressure instead of negative pressure. · Apply epidural anesthesia to decrease the movements in cow. Collection is done with the help of catheter. Three kinds of catheters are used; most commonly used is Foley’s Catheter. It could be two ways or three ways. · It is easily available and inexpensive also. It is soft in nature. In surgery we clamp the uterus horns and throw fluid inside and suck it again which will contain embryos. In non surgical collection we have to fix catheter in uterus horn in a method that embryos cannot pass out of horn into uterus and portion is blocked completely. There are two methods: § Continuous Flow Method: There is less loss of fluid but there are a lot of tubes that we have to handle. It may cause contamination. § Interrupted syringe method: All equipment is disposable so less chance of contamination · When catheters are used, they are sterilized either with ethylene oxide or by color sterilization method. These agents are harmful for embryos. With ethylene oxide it should be sterilized one week before collection and put it in air so that harmful material is decreased. If cold chain is to be used then use normal saline. · Embryo collection is done in diestrus when cervix is closed but still it is penetratable. A steel rod known as cervix dilator is used. All things sterilize. Pass it slowly that will loosen the rings of cervix, take out dilator and pass the catheter. · Catheters are not hard enough because they are of rubber. To create stiffness in catheter we pass steel rod in it and after passing we direct it toward one of the horns. Tip of catheter has opening and an area where there is balloon i.e. deflator. Enter N.S so that balloon is flatted. When the balloon is bigger enough to fill the lumen of the uterus the catheter is fixed. · Now fill the uterus from the opening inside the catheter, the fluid when starts filling in uterus, move the top of horn where the embryos would be and take out the fluid which contains embryos. In first attempt of filling fluid it contains 85 % of embryos. No. of plates are marked. Immediately after the collection, shift the material in lab and then search of embryo. Evaluation Square shaped Petri plates are used. Search with stereomicroscope at 10X. Criteria for Evaluation: · Continuity of zona pellucida (zona is double layered and its diameter is 12-15 µm). · The arrangement of blastomeres in the zona: they should be arranged in circular fashion and no extension of cells from the zona. · Blastomeres should be of uniform size. · Presence of degenerative area: If degeneration present it will appear as black area. It is in %age. · Presence of vacuole. By following the above mentioned criteria we can evaluate the embryos in all grade: Excellent → A: Ideal, spherical, symmetrical with uniform blastomeres, no degenerative area and no vacuole Good → B: Few extrusion of blastomeres, irregular shape, one or two vacuoles Fair → C: Extruded blastomeres, vacuole formation with 19 % degenerative area Poor → D: Numerous extruded blastomeres, more variation in size, more vacuoles, degeneration upto 25 Unfertilized: There will be no cell and just spots present For fresh embryo transfer, embryo upto C grade can be used. If embryo is to be transferred after freezing then only upto B grade embryo can be used. The searching of embryo is done at 10 X but evaluation is done at 50-100 X magnification.
Washing: Loading of Embryo: Suck the embryo washing medium 2-3 cm followed by air column and then second medium column larger than first one i.e. 2.5-3.5 cm having embryo. Again a column of air. Then again column of medium of 2-3 cm and again column of air and then there is open space. Transfer: · Use 1-2 ml of epidural anesthesia and go for examination of ovary to check on which ovary the CL is present. Transfer of the embryo should be transferred into ipsilateral horn (horn containing CL). · Ideal site for the deposition or transfer is 5-10 cm above from bifurcation. · Firstly you have to straighten the horn After transfer that recipient should be kept in observation for the next estrous cycle. If it does not come in estrous then go ultrasonography for pregnancy confirmation.
Short Term Storage: Procedure: For longer period of storage deep freezing is done after loading of straw and then sealed it. Automatic embryo freezing chambers are available. Cryoprotective agents is also added. 10 % glycerol may be used. Seeding: Thawing:
|
Embryo transfer technique in cattle
Embryo transfer technique in cattle
Reference-On request.