Epidemiology of Lumpy Skin Disease in India
Manjunatha Reddy, G.B*., Awadhesh Prajapati., Sanjeevakumar Lalasangi, Viveka Prabhu., Chethan Kumar, H.B., Yogisharadhya R and Shivasharanappa, N
ICAR-National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI), Bengaluru, Karnataka
* corresponding author: gbm.reddy@icar.gov.in
Introduction
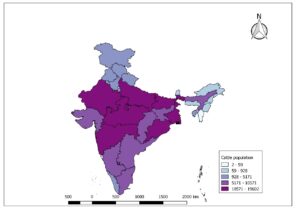
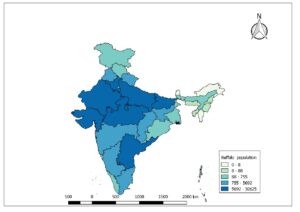
Lumpy skin disease (LSD) is a vector borne pox viral disease of cattle and buffaloes that is characterized by appearance of skin nodules. The natural hosts for this virus are cattle and Asian water buffaloes (Elhaig et al., 2017). Considering the potential for international spread and economic impacts the World Organization for Animal Health (OIE, 2020) classified the disease as notifiable disease. LSD causes huge economic impact on livestock sector due to decreased milk production, loss of draught power, damage to skin, trade restrictions, loss of body condition, abortions, infertility and the cost of veterinary care. The disease was first identified in Zambia in 1929. The LSD has spread from its origin’s in central Africa to the middle east, Europe and Asia with rapid spread occurring since 2013. India reported first time in the year 2019 in Odisha state. The surrounding countries of India viz., Bangladesh, China, Nepal, Bhutan, Myanmar, Sri Lanka, Pakistan have also reported the LSD outbreaks. The whole population of cattle (Fig. 1A) and buffaloes (Fig.1B) in India are at risk of getting infected with this dreadful disease.
Fig.1A: Cattle population at risk in India (19th livestock census)
Fig.1B: Cattle population at risk in India (19th livestock census)
Etiology
The Lumpy Skin Disease is caused by Lumpy Skin Disease virus (LSDV) that belongs to the genus capripoxvirus of the family Poxviridae. LSDV is a brick shaped, enveloped virus with a double stranded DNA (151 kbp). The average size of the virus is 294±20 nm in length and 262 ±22 nm in width (Kitching and Smale 1986). There is only one serotype of LSDV. LSDV is closely related to Sheep pox virus (SPPV) and Goat pox virus (GTPV) but are phylogenetically distinct from each other. All capripoxviruses grow slowly on cell cultures and may require several passages. They can be propagated on a variety of cells of bovine and ovine origin, causing easily recognizable cytopathic effects (CPE). The replication of LSDV occurs in the cytoplasm of the host cell causing intracytoplasmic eosinophilic inclusion bodies. The antibodies produced against one member is serologically cross reactive with the other. Hence, there differentiation among the members of genus capri poxvirus is possible through molecular techniques like PCR and sequencing. LSDV is susceptible to sunlight and detergents containing lipid solvents, but in dark environmental conditions, such as contaminated animal sheds, it can persist for many months (OIE, 2014). The virus can survive for long in cold conditions and as per the current information available, there is no evidence about difference in virulence of the different LSDV strains.
History
The initial description of the LSD clinical indications was made from Zambia, in 1929 (Morris 1931). Up to 1949, there were ongoing reports of disease, which caused severe economic losses in South African nations (Von Backstrom, 1945; Diesel, 1949). Disease first appeared in West Africa in 1974 and East Africa in 1957 (Davies (1991a and b). In a succession of epizootics that were documented, the disease has continued to spread across the majority of the African continent. LSD is now present throughout the Africa (with the exception of Libya, Algeria, Morocco, and Tunisia) (Tuppurainen and Oura 2012). Outside of the sub-Saharan African region, Egypt (1988), Kuwait (1991), Yemen (1995), United Arab Emirates (2000), Bahrain (2003), and Oman (2010) had all reported outbreaks of lumpy skin disease (Tageldin et al., 2014). In India, first outbreaks of lumpy skin was reported in Mayurbhanj and Bhadrak Districts of Odisha in the month of August 2019 (Sudhakar et al., 2020).
Epidemiology
Typically, in endemic countries LSD outbreaks occur in epidemics with several years apart. The existence of a specific reservoir for the virus is not known, nor is how and where the virus survives between epidemics. The presence of growing numbers of naïve animals, abundance of active blood feeding vectors and uncontrolled animal movements are usually the drives for LSD outbreaks. The first outbreak in an unaffected region is usually attributable to either legal or illegal movement of host (cattle/buffaloes). Generally, the long distance jumps are due to host movement and local spread is due to vectors. Outbreaks are usually seasonal and co-inside with abundance of vector population during wet and humid conditions. But, may occur at any time because in many affected regions no season is completely vector free. The morbidity (10-20%) is high and mortality is very low (1-5%). Infected animal eventually clears the infection and there is no scientific evidence for establishment of carrier status in the recovered animals. Even though LSD reported mainly in cattle, the disease has been reported in Asian water buffaloes and African antelope species. Bos taurus is more susceptible to clinical disease than Bos indicus (OIE, 2018). However, even within the herd maintaining the same breed, the clinical manifestation varies from subclinical to fatal disease (Carn & Kitching, 1995). Although sheep and goats co-exist with cattle and buffalo, the role of small ruminant as reservoir of LSDV has not yet been confirmed. have not been implicated as reservoir of LSDV. The role of wildlife in the epidemiology of LSDV requires further investigation. LSDV is not known to infect humans (OIE 2018).
Risk factor
Risk factors for disease occurrence includes effect of agro climate, share of the same grazing and water bodies and unrestricted movement of animals across different borders that would facilitate the spread of outbreaks in various localities. High occurrence of LSD was recorded during wet seasons when biting fly populations are abundant (Table 1). Necrotic skin and air-dried hides are the potential source of virus since the virus persists for month at ambient temperature. Imports of any animal product like meat, milk, or hides originating from a LSD affected country/ies have potential to spread in LSD free countries (Kiplagat et al., 2020). During the infective phase, virus secretes in nasal, lachrymal, pharyngeal secretions, semen, milk and blood and may act as source of infection to others animals (Gumbe , 2018).
| Factors | Variables | Effect |
| Host | Species | · Cattle are more susceptible than buffaloes |
| Sex | · Both sexes are equally susceptible | |
| Age | · Very young and old animal are more suspectable than adults | |
| Breed | · The cross breeds are more susceptible than native Indian breeds however the native breeds are more reactive | |
| Pathogen | Serotype | · Only one serotype
· Not much variation in pathogenicity between strains |
| Stability/viability of virus | · Stable and viable in both dry and cold conditions.
· In biological samples (Blood: 5-16 days, Skin nodule: 39 days, Scabs: several years and semen: 22-42 days) the virus viability varies. |
|
| Environment | Temperature | · LSDV can survive both cold and warm conditions. But, inactivated by heat easily. |
| pH | · Sensitive to both acidic and alkaline pH | |
| Season | · Incidence is more in wet and humid seasons due to abundance of vector population
· Incidence is less during the dry and cold seasons. · Incidence is more during migratory season and livestock fairs or sales or ritual/festivals |
Table.1: Various risk factors and their effect on of the lumpy skin disease.
Lumpy waves in India:
First wave (2019-2020): First outbreaks occurred in Odisha in 2019, where the morbidity was higher and mortality was very low. The disease was also spread to other neighboring states like including all southern states. But the disease was mild and mostly dermal farm of disease was reported in 2019-2020.
Second wave (2020-2021): During this period most of southern states were affected along with other central states but the disease again was mostly of dermal farm and caused many deaths in Maharashtra state compared to other affected states inducing MP and UP.
Third wave (2021-2022): This is ongoing and the disease has turned into more virulent form especially in this wave respiratory form of the disease was more prevalent along with dermal form and maximum mortality was reported from states especially northern India where more of stary or owned cattle population is more. The mortality was also attributable to comorbidities in these stary and unowned or even in animal shelters which are mainly due to managemental practices.
Economic impact
Lumpy skin disease impacts cattle and buffaloes production by causing substantial economic losses. Different stakeholders being affected are animal breeders, dairy farmers, agriculturist (drought power), milk and meat processing and selling industries, wholesale and retail dairy and meat sellers, organic farming community, cattle transport and feed manufacturers etc.,. Among the stakeholder the highest impact will be on landless or marginals poor, small scale and backyard farmers who’s entire enring depends on animal farming only. The economic loses are attributed to decreased milk production, decreased body condition, damage to hide/skin, drought power loss in bullocks, fertility problems and abortions, emaciation, veterinary treatment, secondary complication like maggot wound, mastitis, temporary or parament blindness. Above all the disease also causes mortality (deaths) upto 5% of affected animals which account direct loss. Other costs include national cost of surveillance and control measures like awareness campaigns, monitoring and surveillance program, compensation program for culled or slaughtered animals, cleaning and disinfection of affected farms, sampling and laboratory testing, purchase of vaccines and implantation of a vaccination program, vector control, restriction on movement of live animals and products and carcass disposal. The traders will be affected due to ban on export of live animals and animal products in and out of country.
Transmission
The following are the routes of transmission
- Blood sucking/biting of vectors – Stable fly (Stomoxys calcitrans), Mosquito and Ticks
- Contaminated feed/fodder and water with saliva, nasal and ocular discharges.
- Direct contact with infected animals.
- Vertical transmission: Contaminated semen
- Intrauterine and Trans-mammary
The long-distance jump or transmission of virus is mainly due to movement host and rarely due to transport vehicle carrying the vectors and short distance jump or transmission is due to mainly vectors and other modes of transmission.
Clinical symptoms
The incubation period may very between 4-14 days in experimental conditions, however in field conditions it may go up to five weeks. The clinical sings include nasal and ocular discharge, pyrexia, brisket oedema (Fig 2A), upper limb oedema, drastic reduction in milk production and characteristic skin lesions. Initially, subscapular and pre-femoral lymphnodes become enlarged and are easily palpable (Fig2B). High fever (>40.5) usually precedes the appearance of skin lesions and may be accompanied by a sharp drop in milk yield. The fever may persist for a week. Mild clinical cases may show a few nodules. In severe cases the entire body may be covered with skin lesions (Fig2C). Pneumonia due to secondary bacterial infection is a common sequela. Sometimes ulcerative lesions may also appear in the cornea of the one or both eye, leading to blindness in some cases. Skin nodules usually start to appear within 48 hours of the onset of fever. They are most commonly found at head, neck, udder, genitalia (Fig.2D), perineum and lateral parts of ribcages. Silent or subclinical infections can occur up to one third of infected cattle which do not show any clinical sings although all might have viraemia.
The morbidity and mortality depending the control measures adopted after detection of first case. During the typical course of disease, cattle die several weeks after infection. Thus the outbreak is likely to have been going on for a minimum of 2-3weeks and probably longer. Severe cases of LSD are highly characteristic and easy to recognize or diagnose. However early stages of infection and mild cases may be difficult to distinguish even for the most experienced veterinarians.
Fig.2A: The LSD confirmed animal showing brisket oedema
Fig.2B: LSD affected showing enlarged prescapular lymph node.
Fig. 2.C: LSD affected animal in the mid stage of disease showing various sized nodules all over the body.
Fig.2. D: Breeding bull showing lesions on reproductive organs.
Pathogenesis
The Lumpy Skin Disease (LSD) virus infects the host through the skin or the mucosa of the gastrointestinal system (Sanz-Bernardo et al., 2020). Primary multiplication of virus causes lymphadenitis when it enters the local lymph nodes. Viraemia occurred after the initial febrile reaction and persisted for two weeks. After viremia, virus spread through monocytes and localized in the skin and developed the inflammatory nodules as a result of its rapid multiplication in the cells. LSDV replicates inside the host cells such as macrophages, fibroblasts, pericytes and endothelial cell in the lymphatics and blood vessels walls lead to developing vasculitis and lymphangitis, while thrombosis and infarction may developed in severe cases.Virus multiplication in cells produce hyperplasia and ballooning degeneration of keratinocytes, formation of epidermal micro vesicles, inflammatory cell infiltration into dermis. Four to seven days after infection coalescence of epidermal micro-vesicles occur into large vesicle and ulceration of tissue developed (Namazi, and Tafti, 2021).
Immunity
Young calves, lactating cows, and malnourished animals seem to develop more severe disease that may be due to an impaired humoral immunity (Namazi, and Tafti, 2021). A lifelong cell- mediated immunity is developed in most animals that recover from clinical disease. Calves are born from the infected cow acquire maternal antibodies that may protect them from clinical diseases for approximately six months.
Diagnosis
The early diagnosis of disease is very important for initiating treatment, control and preventive measures. The early and mid stage of LSD is difficult to diagnose by clinical sings. However, the late stage of disease is comparatively easy for clinician to diagnose at field level. Hence, quick and early laboratory diagnosis is very important. The details of suitable samples for laboratory diagnosis are provided in table depending on stage of disease. There are different testes used for virus/antigen and antibody detection in various laboratory depending on the availability of facilities (Table. 1).
| Type of sample/ stage of disease | Early | Mid | Late | All the samples should be shifted to lab at the quickest time possible at 2-80 C
temperature
|
| Swabs (Nasal/lacrimal) | ++++ | +++ | + | |
| Blood | +++ | ++ | – | |
| Skin biopsy | +++ | ++++ | +++ | |
| Scab | + | +++ | ++++ | |
| Serum | – | + | ++ | |
| Milk | – | + | ++ | |
| Rectal swabs/faecal | – | + | ++ | |
| Vectors | + | + | + |
Table.1: Details various clinical samples to be sent for laboratory diagnosis or confirmation in suspected cases of LSD.
Gross and Histopathological findings
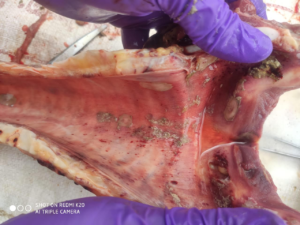
The postmortem examination of the dead animals will reveal grossly the skin nodular lesions all over the body depending the on the severity of the disease. Internally, the respiratory system will show lesions on lungs typical gunshot wounds as overserved in case of sheep and goat pox and even upper respiratory tract will show necrotic pock lesions (Fig 3). Liver may show necrotic foci and digestive system may show pock lesions very rarely. Histopathology of skin shows varied degree of mononuclear cell infiltration especially dendritic cells, lymphocytes and macrophages in dermis and hypodermis. Even though there are reports of presence of intracytoplasmic inclusion bodies in LSD affected organs, we did not observe any inclusions till date which is very peculiar finding especially with respect to LSDV Indian strains.
Fig 3: The trachea upper portion showing pock lesions.
Virus isolation and characterization
Virus isolation from clinical sample is critical in the confirmation of disease. Virus can grow on number of primary cells or cell lines of bovine, ovine, or caprine origin. Chorioallantoic membrane of embryonated chicken eggs and African green monkey kidney (Vero) cells are the most commonly used to grow the virus. Virus grows slowly in cell cultures, and produce the cytopathic effect (CPE) after five to seven days after inoculation. LSDV induces a specific cytopathic effect (CPE) and intracytoplasmic inclusion bodies in cell culture (OIE Terrestrial Manual 2010).
Electron microscopy
Transmission electron microscopic (TEM) can be used for detection of virus directly from clinical sample. Diagnosis can be confirmed within a few hours of receipt of specimens. Mature capripox virions have an average size 320 x 260 nm and are a more oval profile and larger lateral bodies than orthopox virions (OIE Terrestrial Manual 2010).
Molecular detection methods
With advancement of molecular techniques like PCR, diagnostic of LSD become easy and specific. The molecular testing is critical for monitoring the spread of these viruses and controlling disease outbreaks. LSD virus confirmation may be done utilizing a Capri poxvirus specific PCR approach on conventional (Zheng et al., 2007) and real-time PCR technologies (Bowden et al., 2008). For differentiating virulent LSDV from the vaccine strain, Restriction Fragment Length Polymorphism (RFLP) has also been used (Menasherow et al., 2014). Molecular assays employing loop-mediated isothermal amplification to identify capripoxvirus genomes have been shown to have sensitivity and specificity comparable to real-time PCR, with a simpler approach and a cheaper cost.
Serological tests
The only serologically approved test available is the Virus neutralization test (VNT) for monitoring of LSDV antibodies (Milovanović et al., 2019). Besides Indirect fluorescent antibody testing (IFAT), viral neutralization, enzyme-linked immunosorbent assays (ELISA), and immunological blotting (Western blotting) are used in different research laboratory (Namazi, and Tafti, 2021). Neutralizing antibodies occur 3-4 days after the onset of clinical symptoms and reach maximal titer levels in 2-3 weeks. The agar gel immune diffusion test (AGID) and IFAT are less specific than VNT due to cross-reactivity with antibodies to other poxviruses. Western blotting is sensitive and specific, but it is difficult and expensive to perform.
Differential diagnosis
The differential diagnosis of LSD includes pseudo-LSD, dermatophilosis, dermatophytosis, bovine farcy, photosensitisation, actinomycosis, actinobacilosis, urticaria, insect bites, besnoitiosis, nocardiasis, demodicosis, onchocerciasis, pseudo-cowpox, and cowpox, foot and mouth disease, bovine viral diarrhoea, malignant catarrhal fever, infectious bovine rhinotracheitis, and bovine popular stomatitis (OIE, 2018). In case, if homologues vaccination is practiced the vaccine induced mild disease should be considered for differential diagnosis.
Treatment
There is no specific treatment to treat Lumpy Skin Disease. The treatment is by means of supportive care. The following protocols may be followed in case of LSD outbreak.
- Separation and isolation of suspected/affected animals.
- Symptomatic treatment of affected animals. The following table may be taken for preparing the treatment schedule based the experience and availability of medicine in the locality.
| Form of disease | Antibiotic (SA) | Antibiotic (LA) | Antihistamine | Pain killer | Multivitamin | Mineral | Immunity booster | Herbal or home remedies | Deworming | Vector control | Anti haemoprotozoan |
| Dermatitis | + | ++ | +++ | +++ | + | + | + | ++ | Once | +++ | After lab confirmation |
| Dermatitis +Respiratory | +++ | +++ | +++ | ++ | ++ | ++ | ++ | +++ | +++ | ||
| Generalised | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ |
3.Feeding management: Give easily digestible preferably liquid feed/food and succulent green fodder.
4.Proven or indigenous technology knowledge based herbal preparations may be used for wound and dermatitis treatment, as immunomodulators and antioxidants and fly repellents,
Immunization
As the LSDV is quite stable and can remain for months, a long-term vaccination regimen should be developed and made mandatory to control LSD covering the entire bovine population. A live attenuated vaccine has been developed and used in endemic country to control the disease. Members of the capripoxvirus are known to provide cross-protection. Hence, homologous (Neethling LSDV strain) and heterologous (sheeppox or goatpox virus) live attenuated vaccines can all be used to protect cattle (OIE, 2013). Most of the vaccine manufacturer in African countries use Neethling strain for virus for production LSD vaccine. This LSD vaccine is attenuated by culturing sixty times in lamb kidney cells followed by twenty times sub-culturing in chorio-allantoic membrane of embryonated chicken eggs. Attenuated virus provides immunity for three years after administration in bovines. Dose of vaccine varies from manufacturer to manufactures generally from 102.5 to 104 TCID50/dose. Attenuated LSDV vaccinations may produce minor adverse reaction known as the “Neethling response. Besides use of capripox virus vaccines in bovines in also recommended due to cross protective nature of vaccine. SPPV vaccines at a higher dose (three, five and ten-fold) have been used in cattle against LSDV in those regions where LSD and SPP are both present. Kenyan or Romanian sheep pox virus strains have been used to confer LSD immunity in bovines. These live vaccines could potentially serve as a source of infection for the susceptible sheep and goat populations, hence they are not recommended in nations free of sheep and goat pox. In India as per the guideline of Department of Animal Husbandry and Dairying, Ministry of Fisheries, Animal Husbandry and Dairying goat pox vaccine (Uttarkashi strain) is used @103.0 TCID per animal subcutaneously. Recently India has also developed the LSD vaccine from local strain named Lumpi-ProVacInd. The vaccination plan should be chosen depending on the needs of the country and the resources that are available. Animals that have just been introduced to a farm should be immunised. Similar to this, calves from cows who have received a vaccination or have recovered from a natural infection need to receive one between the ages of three and four months. Vaccinations can be administered annually to all susceptible cattle and buffloes especially in high risk groups and areas.
Prevention and control
Like several other viral diseases of livestock, the LSDV can be effectively controlled by implanting the following measures.
- Animal movement restriction: the animal movement in or out from infective zone should be banned. If possible, the live animal markets, fairs, gatherings, migration etc., should be stopped.
- Preventive vaccination: The ring vaccination (5km radius) should be carried in all (cattle and buffaloes) healthy animals which are aged above 4 months as per the GoI guidelines with goat pox vaccine (100 TCID50).
- Bio-security measures: The bio-security measures like quarantine, isolation, disinfection of premises, deworming, disposal of farm waste and carcasses, people movement, insemination, health records etc., should be regularly practiced/monitored.
- Vector Control: The measures should be adopted for control of biting or blood feeding vectors through application of insecticide or repellents and also through reducing the breeding grounds for these vectors in farm premises.
- Cleaning and disinfection: The affected areas including the farm equipments and fomites should be cleaned then disinfected with appropriate chemicals / disinfectants like ether (20 %), chloroform, formalin (1 %), phenol (2 % for 15 minutes), sodium hypochlorite (2-3 %), iodine compounds (1:33 dilution) and quaternary ammonium compounds (0.5 %).
- Creating awareness among different stake holders through radio, TV, press releases, posters, newspapers / print media, digital media, social media posts, webinars, workshops etc.,
- Prepare SOPs at state/regional level
- Prepare and train the rapid response teams
- Keep networking with line departments for better and quick response.
- Monitoring and surveillance especially in unaffected areas.
References
- Bowden, T. R., Babiuk, S. L., Parkyn, G. R., Copps, J. S., & Boyle, D. B. (2008). Capripoxvirus tissue tropism and shedding: A quantitative study in experimentally infected sheep and goats. Virology, 371, 380–393.
- Das, A., Babiuk, S. and McIntosh, M.T., 2012. Development of a loop-mediated isothermal amplification assay for rapid detection of capripoxviruses. Journal of Clinical Microbiology, 50(5), pp.1613-1620.
- Davies, F.G., 1991. Lumpy skin disease, an African capripox virus disease of cattle. British Veterinary Journal, 147(6), pp.489-503.
- Diesel, A.M., 1949. The epizootology of” lumpy skin disease” in South Africa.
- Elhaig, M.M., Selim, A. and Mahmoud, M., 2017. Lumpy skin disease in cattle: Frequency of occurrence in a dairy farm and a preliminary assessment of its possible impact on Egyptian buffaloes. Onderstepoort Journal of Veterinary Research, 84(1), pp.1-6.
- Gumbe, A.A.F., 2018. Review on lumpy skin disease and its economic impacts in Ethiopia. Dairy Vet. Anim. Res, 7(2), pp.39-46.
- Kiplagat, S.K., Kitala, P.M., Onono, J.O., Beard, P.M. and Lyons, N.A., 2020. Risk factors for outbreaks of lumpy skin disease and the economic impact in cattle farms of Nakuru County, Kenya. Frontiers in veterinary science, 7, p.259.
- Kitching, R.P. and Smale, C., 1986. Comparison of the external dimensions of capripoxvirus isolates. Research in Veterinary Science, 41(3), pp.425-427.
- Menasherow, S., Rubinstein-Giuni, M., Kovtunenko, A., Eyngor, Y., Fridgut, O., Rotenberg, D., Khinich, Y., & Stram, Y. (2014). Development of an assay to differentiate between virulent and vaccine strains of lumpy skin disease virus (LSDV). Journal of Virological Methods, 199, 95–101.
- Milovanović, M., Dietze, K., Milićević, V., Radojičić, S., Valčić, M., Moritz, T. and Hoffmann, B., 2019. Humoral immune response to repeated lumpy skin disease virus vaccination and performance of serological tests. BMC veterinary research, 15(1), pp.1-9.
- Morris, J.P.A., 1930. Pseudo-urticaria. Northern Rhodesia. Dept Anim Health Ann Rpt, 12.
- Namazi, F. and Khodakaram Tafti, A., 2021. Lumpy skin disease, an emerging transboundary viral disease: A review. Veterinary Medicine and Science, 7(3), pp.888-896.
- OIE, Office International des Epizooties. (2017). Manual of diagnostic tests and vaccines for terrestrial animals 2017. Chapter 2.4.13 Lumpy skin disease. Retrieved from http://www.oie.int/fileadmin/Home/ eng/Health standards/tahm/2.04.13_LSD.pdf
- OIE, Office International des Epizooties. (2017). Manual of diagnostic tests and vaccines for terrestrial animals 2017. Chapter 2.4.13 Lumpy skin disease. Retrieved from http://www.oie.int/fileadmin/Home/ eng/Health_standards/tahm/2.04.13_LSD.pdf.
- (2013). World Organization for Animal Health. Lumpy Skin Disease.
Technical Disease Card. - Sanz-Bernardo, B., Haga, I.R., Wijesiriwardana, N., Hawes, P.C., Simpson, J., Morrison, L.R., MacIntyre, N., Brocchi, E., Atkinson, J., Haegeman, A. and De Clercq, K., 2020. Lumpy skin disease is characterized by severe multifocal dermatitis with necrotizing fibrinoid vasculitis following experimental infection. Veterinary pathology, 57(3), pp.388-396.
- Sudhakar, S.B., Mishra, N., Kalaiyarasu, S., Jhade, S.K. and Singh, V.P., 2022. Genetic and phylogenetic analysis of lumpy skin disease viruses (LSDV) isolated from the first and subsequent field outbreaks in India during 2019 reveals close proximity with unique signatures of historical Kenyan NI‐2490/Kenya/KSGP‐like field strains. Transboundary and Emerging Diseases, 69(4), pp.e451-e462.
- Sudhakar, S.B., Mishra, N., Kalaiyarasu, S., Jhade, S.K., Hemadri, D., Sood, R., Bal, G.C., Nayak, M.K., Pradhan, S.K. and Singh, V.P., 2020. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transboundary and Emerging Diseases, 67(6), pp.2408-2422.
- Tageldin, M.H., Wallace, D.B., Gerdes, G.H., Putterill, J.F., Greyling, R.R., Phosiwa, M.N., Al Busaidy, R.M. and Al Ismaaily, S.I., 2014. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Tropical animal health and production, 46(1), pp.241-246.
- Tuppurainen, E., Alexandrov, T. and Beltrán-Alcrudo, D. (2017). Lumpy skin disease field manual – A manual for veterinarians. FAO Animal Production and Health Manual No. 20. Rome. Food and Agriculture Organization of the United Nations (FAO). 60 pages.
- Tuppurainen, E.S.M. and Oura, C.A.L., 2012. lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transboundary and emerging diseases, 59(1), pp.40-48.
- Von Backstrom, U., 1945. Ngamiland cattle disease: preliminary report on a new disease, the etiological agent being probably of an infectious nature. Journal of the South African Veterinary Association, 16(1), pp.29-35.
- World Organisation for Animal Health OIE, (2020). OIE-Listed diseases, infections and infestations in force in 2020. Available from https://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2020/. Accessed on 17 November 2020.
- Zheng, M., Liu, Q., Jin, N. Y., Guo, J. G., Huang, X., Li, H. M., Zhu, W., & Xiong, Y. (2007). A duplex PCR assay for simultaneous detection and differentiation of Capripoxvirus and Orf virus. Molecular and Cellular Probes, 21, 276–281.
****