FAQs ON RABIES (26-100) FOR VETERINARIAN
by-DR RAJESH KUMAR SINGH ,JAMSHEDPUR,JHARKHAND, INDIA,
9431309542,rajeshsinghvet@gmail.com
All veterinarians, from those who work with farm animals to pets to wild animals, play a crucial role in protecting human health as well by managing crises such as diseases and zoonoses and foodborne diseases. Cross-border collaboration, communication and cooperation between veterinarians, represented by various vet org, and the various animal and human health-focussed agencies and industries they work with, such as the OIE, FAO and WHO, are important parts of global health initiatives-ONE WORLD ONE HEALTH..
The work these veterinarians undertake has been integral to the containment and eradication of several major diseases that have harmful effects on both human and animal health. One of the most well-known cases is rabies, which can be deadly in both humans and animals. The responsibility for controlling rabies and preventing transmission of this deadly viral disease is associated with vets. Vets are being asked several questions from the common people of society about Rabies.By providing detail knowledge about rabies , we can make our people aware about the deadly Rabies. On the occasion of the WORLD RABIES DAY I.E 27TH OCTOBER , I thought to post some most crucial informations which are not only useful to common people but also to vets.In this post I have compiled 155 faqs on behalf of common people from vets, It is not possible to post all at a times so it will be posted subsequently
26.Is there any seasonal variation in dog bite cases?
• Maximum dog bites were observed in the autumn months. It is observed that there is an increase during warm-weather months (May through August) and a corresponding decrease during colder months (November through March).
27.What are the myths about rabies in India?
• Following myths about rabies are very much prevalent in India:-
1. In India, there are many myths and wrong practices concerning the management of an animal bite. People apply turmeric, salt and sometimes ghee over the wound area. Chilies, hydrogen- peroxide and cow dung are some other wrong practices followed mainly in the rural parts of India.
2. Some herbal extracts will cure rabies.
3. In rural areas, people also resort to witchcraft and religious practices.
4. Washing of wound(s) can cause hydrophobia.
5. Dietary changes can cure, that is, shift from vegetarianism to non-vegetarianism or vice versa; stopping consumption of white things etc.
6. A single dose vaccine will prevent rabies.
7. Vaccines are more effective if taken on empty stomach.
8. One should not take bath; eat meat and eggs during vaccination.
9. Gems and stones have magical properties against rabies.
28.Is there any specific treatment for clinical rabies?
• There is no specific treatment for clinical rabies. Key to survival after exposure to rabies virus is administration of post-exposure prophylaxis (PEP) as soon as possible. Death is virtually inevitable once clinical signs develop. Medical management is supportive and palliative
29.What are most dangerous sites of bites/exposure in man?
• Theoretically, the richly innervated areas like head, neck, face, hands and genitals are the most dangerous sites of bite in man. But practically, it is often the wounds on legs, which are ignored/neglected, that cause rabies.
30.What does post-exposure prophylaxis (PEP) include?
• It includes:-
(1) Local treatment of wound(s)
(2) Categorization of animal bite wound(s)
(3) Anti Rabies Vaccination. (ARV)
(4) Administration of Rabies Immunoglobulin (RIG)
(5) In addition, Tetanus prophylaxis, analgesics & systemic antibiotics may be given.
31.What are the contraindications to post exposure prophylaxis?
• As rabies is 100% fatal, there are no contraindications to post exposure prophylaxis.
32.Whether washing of animal bite wound(s) is essential?
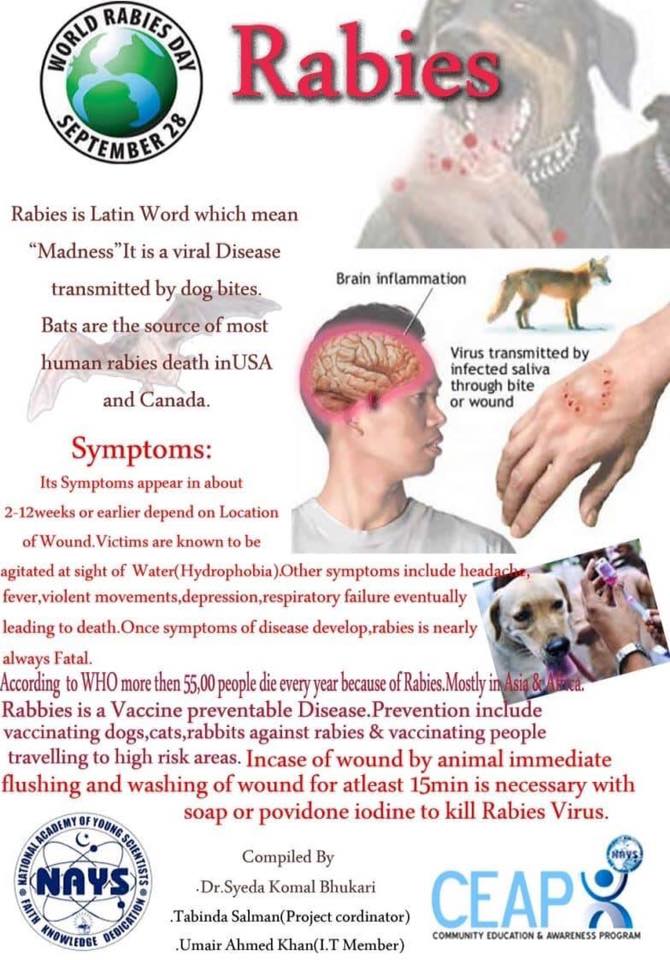
• By mere washing of wounds and application of antiseptics, the risk of rabies will reduce by about 50%. The maximum benefit of the wound washing is obtained when fresh wound is cleaned immediately. It is important to remove saliva containing rabies virus at the site of bite by physical or chemical means. This can be done by prompt and gentle thorough washing with ordinary soap or detergent and flushing the wound with running tap water for at least 15 minutes.
Washing of the wound must be done as long as the wound is raw; irrespective of the time elapsed since the exposure. Care must be taken not to disturb the scab, if formed.
After washing with water & soap, disinfectants like Povidone Iodine or Surgical Spirit must be applied.
In extraneous circumstances, other alcoholic (>40%) preparations like Rum, Whisky or after-shave lotion may be applied on the wound. If soap or antiviral agent is not available, the wound should be thoroughly washed with water.
33.Can we apply local antibiotics or antimicrobial agents on the site of bite?
• After cleansing of the bite wounds, local antimicrobial agents can be applied. Discourage local wound applicants like turmeric, neem, red chili, lime, plant juices, coffee powder etc.
34.Can the animal bite wounds be cauterized?
• Cauterizing the wound is not advisable as it leaves a very bad scar and also does not confer any additional advantage over washing the wound with water and soap. It amounts to malpractice and the doctor can be sued for compensation under COPRA.
35,Whether the dog bite wound should be allowed to bleed, bandaged, or stitched?
• Do not bandage the wound as far as possible and if unavoidable, apply non-adherent, absorbent dressings (paraffin gauze or Melolin) to absorb the discharge from the wound. Tincture iodine should not be used.
Avoid Suturing of the bite wound as a rule since it may risk inoculation of the virus deeply into the wound. However, if the wound has to be sutured, it should be done as late as possible from several hours to 3 days after infiltration of RIGs. If RIGs is not available, as a last resort, the wound must be flushed with povidone iodine before suturing. The suture should be loose and not interfere with free bleeding and drainage.
Human and animal bite wounds are best closed by secondary sutures after one week and after proper cleansing and daily wound care. Primary surgical intervention must be avoided if possible.
36.Can the wound be deepened for cleaning purpose?
• We should never try to deepen the bite wound. Deepening of wound for cleaning depends on area of injury, extent of injury and the aim should be to preserve as much tissue as possible and to excise dead tissue only.
37,How common are animal bites and infections?
• Only 15 to 20 percent of dog bite wounds become infected. Puncture wounds and hand wounds are more likely to become infected than scratches
38.Is there any role of systemic antibiotic treatment for animal bites infections?
•
Using antibiotics may be helpful, particularly in high-risk wounds such as those of the hand.
Antimicrobials effective in the empiric treatment of patients with animal bite and human bite wounds
Animal bite Human bite
Amoxicillin-clavulanate (PO) + +
Ampicillin-sulbactam (IV) + +
Moxifloxacin (PO, IV) + +
Gatifloxacin (PO, IV) + +
Doxycycline (PO, IV) + +
Most mammalian bites are caused by dogs, cats or humans. Cat and human bites often become infected, so antibiotic prophylaxis should be considered in addition to wound management. Amoxycillin with clavulanate is suitable for prophylaxis in most cases. Prophylaxis is usually continued for 5–7 days.
WHO Guide for treatment against rabies post-exposure:-
Category (Type of contact with a suspect or confirmed rabid domestic or wild animal, or animal unavailable for observation) Recommended treatment.
I. Touching or feeding of animals, Licks on intact skin None, if reliable case history is available
II. Nibbling of uncovered skin, Minor scratches or abrasions without bleeding, Administer vaccine immediately. Stop treatment if animal remains healthy throughout an observation period of 10 days or if animal is killed humanely and found to be negative for rabies by appropriate laboratory techniques.
III. Single or multiple transdermal bites or scratches, Contamination of mucous membrane with saliva (i.e. licks), Licks on broken skin. Administer rabies immunoglobulin and vaccine immediately. Stop treatment if animal remains healthy throughout an observation period’ of 10 days or if animal is killed humanely and found to be negative for rabies by appropriate laboratory techniques.
When in doubt of degree of exposure to rabies risk, it is safer to over treat than under treat.
39.Is it important to elicit information about biting animal?
• It is very important to elicit information about biting animal.
Low risk category includes healthy pets and regularly vaccinated dogs/cats.
Moderate risk category includes healthy pets but vaccination status doubtful or not done.
High risk category includes rabid, sick, died, stray dogs/cats, or wild animals.
40.What are the various types of modern anti-rabies vaccines?
• These are:-
(1) Purified vero cell rabies vaccine (PVRV)
(2) Purified chick embryo cell vaccine (PCECV)
(3) Human diploid cell vaccine (HDCV)
(4) Purified duck embryo vaccine (PDEV)
41.Does strain of virus used in rabies vaccine play a role in eliciting the required response?
• Response to rabies vaccination is a unique mix of specificities to rabies virus antigens. Antibodies develop out of intrinsic host responses and in response to extrinsic factors such as the amount of antigen given, type of antigen, and route of exposure or vaccination. There may be substantial variation in the neutralizing activity and quantity of rabies virus neutralizing antibodies (RVNA) produced. Therefore, the strain of virus used in antirabies vaccine plays a role in eliciting the required response
42.Is there any advantage of PCEC vaccine with Pitman-Moore (PM) strain?
• Compared to other continuous cell lines based rabies vaccines, PCEC vaccine with Pitman-Moore (PM) strain has several advantages, these includes:
1. This method provides vaccine with high yield, greater potency and immunogenicity which makes the vaccine comparatively cost effective and unique.
2. It is more readily scalable to large scale commercial vaccine production
43.Why do some people fear for vaccine?
• In the past, rabies vaccine was a sheep brain derived Nerve Tissue Vaccine (NTV) and used to have considerable side effects. Large volumes and number of injection were required. So, rabies vaccine acquired the bad reputation of a dangerous vaccination. However, these fears are no longer justified with modern rabies vaccines that are very safe.
44.A person started with PVRV wants to change over to HDCV or PCECV or PDEV or vice versa. What should be done?
• Of the currently available TCVs such as HDCV, PCECV, PVRV, and PDEV, all are equally good and approved by WHO. All are interchangeable following non availability of one brand or due to allergy to one of the CCVs or PDEV. All are considered protective throughout the world against different strains of rabies viruses in different parts of the world.
45.Can modern vaccines be diluted with tetanus toxoid or water or any other diluents and injected?
• The modern anti-rabies vaccines should not be diluted with tetanus toxoid or any other diluents other than that provided with the vaccine
46.Can a rabies vaccine be given to a pregnant woman?
• Following animal bite, rabies vaccine can be given to a pregnant woman. Medical termination of pregnancy should not be done as a routine clinical practice.
47.Can a lactating mother be given antirabies vaccination?
• Modern Anti-rabies vaccines being used now are Tissue Culture Vaccines (TCV) inactivated by beta-propiolactone (BPL) and can safely be given to a lactating mother.
48.Is there a one-shot ARV? Is there any ARV that offers lifelong protection?
• There is no single dose vaccine or a vaccine that gives lifelong immunity.
49.Whether newborn or neonates or infants require smaller volume/lesser dosage of rabies vaccine?
• All modern rabies vaccines have a uniform dosage for all age groups.
50.Can rabies vaccine be given to a child with chicken pox or measles?
• As rabies is 100% fatal, there is no contraindication for anti-rabies vaccination. Rabies vaccine can be given to a child with chicken pox or measles and it is effective. If possible administration of measles vaccine should be postponed by a fortnight after the completion of antirabies immunization.
51.Can rabies vaccine be given to HIV or AIDS patient?
• Rabies vaccine can be given to HIV or AIDS patient. It is recommended to administer RIGs even in category II exposures in such patients and double dose of anti-rabies vaccine must be given on day 0 of vaccination.
52.Can rabies vaccine be given to a patient with jaundice?
• Rabies vaccine can be given to a patient with jaundice.
53.Can a person receiving/completed antirabies immunization, either pre-exposure or post-exposure donate blood?
• A person receiving/completed antirabies immunization can donate blood. However, the recipient does not benefit from the transfer of rabies-neutralizing antibodies due to hemodilution.
54.Can “major surgery” be conducted after the dog bite?
• There is no contraindication for any surgery along with anti-rabies vaccination and a full course of anti-rabies immunization should be given, irrespective of the surgery or other procedures.
55.What is “window period”?
• It is the time taken by Anti-Rabies vaccine to produce protective levels of antibodies in the patient. The window period is of 7-14 days
56.What is the criterion for “protection” after immunization?
• The criterion for protection after immunization is that the rabies virus neutralizing antibody (RVNA) titer of > or = 0.5 IU/ml of serum in the vaccinated person is considered protective.
The facility for this test is available at NCDC, Delhi, CRI, Kasauli, Pasteur institute, Coonnor, NIV, Pune and NIMHANS, Bangalore
57.What is the “potency’ of rabies vaccine?
• WHO recommends that the vaccine potency should be at least 2.5 IU per dose. The potency is the capacity of the vaccine to induce immune response
58.Can modern rabies vaccines be stored at room temperature?
• Vaccine must be stored at +20 C to + 80 C. It should not be stored at room temperature. Besides, the vaccine should not be exposed to sunlight and heat.
59.What are the consequences of a vaccine with a higher potency?
• There are no risks with vaccine with a higher potency.
60.What is the post-exposure (WHO ESSEN-IM) Schedule?
• The post-exposure (WHO ESSEN-IM) Schedule is I.M. vaccination on days 0(day of first dose of vaccine and not the day of bite), 03, 07, 14 and 28.
Vaccine should be injected deep into deltoid muscle (in Adults) or antero-lateral aspect of thigh (in Children).
61.Can the vaccine be injected in gluteal region?
• Rabies vaccine must not be administered in gluteal region as the gluteal fat may retard vaccine absorption resulting in delayed and lower seroconversion
62.If a person is on antimalarials or steroids or taking immunosuppressant drug, what is the schedule?
• The vaccine on day 0 (first injection) must be doubled and given at two sites (deltoids or thigh in young children). In category II exposures, it is recommended to administer even RIGs along with vaccine. Rest of the schedule is same as for any other patient.
63.A previously immunized person is bitten again. What is the re-exposure immunization schedule?
• Only two doses of vaccine at days 0 & 3 are required. RIGs are not required (WHO 2007). However, in laboratory confirmed rabies exposures, irrespective of past rabies immunization, full course of PEP and RIGs is recommended. In rabies, it is safer to over treat than under treat.
64.What should be done with a patient who has had exposure but goes for treatment after considerable delay (weeks to months)?
• An early and correct administration of modern anti-rabies vaccine is life saving. The vaccination must be started immediately irrespective of the status of the biting animal. Patients who seek treatment after a delay of 48 hours or even months after having been bitten should be dealt in the same manner as if the exposure occurred recently.
65.A pregnant woman develops rabies. What should be done?
• In case the mother develops rabies, the rabies virus is not known to cross placental barrier, and as a result, the fetus is safe. Hence, the pregnant woman with rabies should be clinically managed and if induction of pregnancy or caesarean section is possible, the obstetrician should do it with some “personal precautions” and immuno-prophylaxis (usually three doses of modern vaccine or if any accidental exposure is there, then full course of post-exposure vaccination either by IM or ID route should be given to the obstetrician.) Later the new born may be given full course of rabies PEP vaccination.
66.Is it essential to perform an antibody test on the patient following antirabies vaccination?
• Antibody tests, that is, rapid fluorescent focus inhibition test (RFFIT), mouse neutralization test (MNT) are done only at selected few reference centers in India. Antibody tests are not required on a routine basis following antirabies vaccination if vaccination is correct and reliable.
67.Whether observing the dog for 10 days without initiating treatment is risky or justifiable?
• Observing the dog for 10 days without initiating treatment is risky and not at all justifiable. It is mandatory to start treatment soon after exposure. The vaccination must be started immediately irrespective of the status of the biting animal. Improper treatment to animal bite victims may lead to rabies death and litigation under Consumer Protection Act (COPRA). According to Consumer Protection Act, the animal bite should be considered as “medical urgency” and treated with due care.
68.Is there carrier state of rabies in dogs?
• Carrier state of rabies in dogs/cats is not yet conclusively proven and established.
69.A patient received two doses of modern vaccine (on days 0 and 3) and the dog was well on days 5 and 7 (third injection due, but not given). However, the dog dies on any day between 8 and 15. What should be done?
• In case, day 0 and 3 inj. were given and inj. due on day 7 was postponed because the dog was kept under observation but the dog dies between 8 and 15 days, three doses of vaccine must be given as close to the original dates of the schedule and all five inj. must be completed by day 28.
70.How to approach a case of irregularities in treatment schedule, e.g., if patients missed the doses as per the due dates, which means if the schedule is broken?
• First three doses of modern rabies vaccine must be very timely and for the fourth and fifth, one or two days of variation is permissible.
71.As there are different strains of rabies viruses in different parts of the worlds, whether all antirabies vaccines are effective against all these strains of viruses?
• All anti-rabies vaccines are considered protective against different strains of rabies viruses in different parts of the world.
72.Is there any dietary restriction during PEP?
• It is advisable to abstain from alcoholic drinks during the course of rabies vaccination as it may affect the immune response.
73.What is Pre-exposure vaccination?
• Pre-exposure (Prebite) vaccination means immunization before the bite.
74.What is I.M. pre-exposure vaccination schedule?
• The regimen is three IM injections on days 0, 7 & 28.
75.What is the importance of pre-exposure vaccination?
• Pre-exposure vaccination simplifies post-exposure vaccination because after bite, those who have received full Pre-exposure vaccination, only two doses of vaccine at days 0 & 3 are required. RIG is not required (WHO 2007).
76.Can a pregnant woman be given pre-exposure vaccination?
• Although no teratogenicity or other adverse effects are reported with modern rabies vaccines, pre-exposure vaccination should be avoided in pregnant woman.
77.What drugs are contraindicated during rabies immunizations?
• Immunosuppressive drugs, such as steroids, antimalarials, anticancer drugs are generally contraindicated during rabies immunizations. However if these drugs cannot be avoided, then day 0 dose of vaccine should be doubled.
78.What is the dose and schedule of IDRV?
• For PEP, the modified “TRC ID” schedule (2-2-2-0-2) is the only schedule approved by the DCGI at present. ID vaccine is given on days 0, 03, 07, and 28.
The dose of each ID shot has been specified to be of 0.1 ml. of the permitted vaccines.
For pre-exposure vaccination, 0.1 ml of ID-approved vaccine is to be given ID over one deltoid on days 0, 7 and 21 or 28 days.
79What is eight-site intradermal regimen (“8-0-4-0-1-1” regimen)?
• One dose of 0.1 ml is administered intradermally at eight different sites (Either upper arms, lateral thighs, supra-scapular region, and lower quadrant of abdomen) on day 0. On day 7, four 0.1 ml injections are administered intradermally into each upper arm (deltoid region) and each lateral thigh. Following these injections, one additional 0.1 ml dose is administered on days 28 and 90. This regimen lowers the cost of vaccine administered by intramuscular regimens and generally produces a higher antibody response than the other recommended schedules by day 14. It does not result in a significantly earlier antibody response and in order to ensure optimal treatment, a passive immune product must be administered to patients presenting with severe exposures.
However, it is not approved for use in India by DCGI.
80.dose of Verorab (PVRV) and Abhayrab (PVRV) is 0.5mL; that of Rabipur (PCEC) and PVRV (Coonoor) is 1mL. Still is the ID dosage of all vaccines uniformly 0.1mL?
• The ID dosage of all approved vaccines is uniformly 0.1 mL per ID site irrespective of their IM dosage.
81.What is the mode of action of IDRV?
• It is deposition of approved modern rabies vaccine (or antigen) in the layers of dermis of skin by which the immuno-receptive Langerhan cells present within the dermis are stimulated. Subsequently the antigen is carried by antigen presenting cells via the lymphatic drainage to the regional lymph nodes and later to the reticulo-endothelial system eliciting a prompt and highly protective antibody response. Immunity is believed to depend mainly upon the CD 4 + T- cell dependent neutralizing antibody response to the G protein. In addition, cell-mediated immunity has long been reported as an important part of the defense against rabies. Cells presenting the fragments of G protein are the targets of cytotoxic T- cells and the N protein induced T helper cells. The immune response induced by IDRV is adequate and protective against rabies.
82.Can all available anti-rabies vaccines be used by intra-dermal route?
• Not all vaccines produced in India are at present fit for Intra-dermal usage. The following vaccines have been approved by DCGI for use by intra-dermal route.
(1) Purified chick embryo cell vaccine (PCEC) – Rabipur and Vaxirab-N.
(2) PVRV (Purified verocell rabies vaccine) – Verorab-vial of 0.5 ml,
(3) PVRV – Abhayrab – vial of 0.5 ml., Human Biological Institute
(4) PVRV – Indirab, vial of 0.5 ml/1.0 ml. Bharath Biotech, Hyderabad.
PDEV (Vaxirab) and HDCV (Rabivax) are approved for IM use only and not for IDRV. Rabies vaccines formulated with an adjuvant should not be administered intradermally.
83.Can the type of vaccine be interchanged during the course of IDRV?
• As far as possible, the same vaccine should be used throughout a course of IDRV. However, in exigencies, the permitted vaccines are interchangeable.
84.Can the routes of vaccination IM and ID be used interchangeable?
• It is recommended by many internationally reputed experts that the phenomenon of mixing of IM and ID schedules is not to be practiced and must be avoided as far as possible.
85.What should be done if the ID injection fails, that is, spills out or goes subcutaneous?
• WHO recommends that in cases where the wheal over the skin is not formed then the patient should receive another dose of vaccine at a site nearby. If the IDRV fails in any one of the sites after two attempts, then the vaccine must be given by IM route and the remaining doses of the schedule given by IM route only.
86.If for some reason, IDRV cannot be given in deltoid region, what are the alternative sites?
• IDRV can be given in deltoid region, supra-scapular, anterior abdominal wall and the upper part of thigh
87.What are the common side effects of intra-dermal rabies vaccination?
• Cell culture vaccines have proved remarkably safe and free of significant adverse events. However, mild symptoms of pain, erythema, irritation or swelling at the intradermal injection sites occur in 3% to 92% of patients. The most frequent symptom is local irritation in 7% to 64% of vaccines. Generalized symptoms reported by 3% to 14% of recipients include headache, fever and influenza- like illness. Transient macula papular and urticarial rashes are occasionally seen. All these adverse effects are mild, transient and self limiting and rarely call for the use of anti-histamines (tablet or syrup Avil) and analgesics.
88.What precautions should be taken while vaccinating by IDRV route?
• 1. The ID injections must be administered by staff trained in this technique.
2. The Vaccine vials must be stored at +20 C to + 80 C after reconstitution and
3. The total content should be used as soon as possible, but at least within 8 hours.
4. The 0.1 ml. ID administration of cell-culture vaccine should create a wheal of at least 5 mm diameter with “peau de orange” appearance.
5. If ID dose is given sub-cutaneously then there is a possibility of poor immune response due to low antigen load. This may be life threatening.
89.Are there any dietary restrictions during IDRV?
• There are no dietary restrictions during IDRV. However, alcohol may be avoided as it may affect the immune response.
90.Is sera testing of IDRV patients for antirabies antibodies necessary as a measure of knowing its efficacy?
• Routine sera testing for rabies antibodies to know its efficacy is not required.
91.Whether pregnancy and lactation are contraindications for IDRV?
• Pregnancy and lactation are not contraindications for IDRV.
92.Is there a need to alter the dose or schedule of any concomitant medication during IDRV?
• There is no need to alter the dose or schedule of any concomitant medication during IDRV.
93.Can IDRV be given in private hospital?
• The ID route has been permitted to be used in selected anti-rabies clinics (ARCs) having an adequate number of patients (at least 5/day) seeking post-exposure prophylaxis against rabies every day to make IDRV viable and cost-effective.
94.A previously immunized person is bitten again. What is the re-exposure IDRV schedule?
• Following re-exposure the bitten person needs only two doses of 0.1 ml. of ID dose at one site only on Day 0 and day 3 and RIGs are not needed. Proper wound treatment is very important.
95.Can IDRV be given to infants whose skin is thin?
• For children below 5 years of age, it is advisable to adopt IM vaccination
96.What is the potency requirement of IDRV?
• Only three countries are practicing IDRV in regular patients attending regular anti-rabies clinics (ARCs) for more than ten years. These countries are Thailand, Philippines and Sri Lanka.
In Thailand and Sri Lanka, the potency requirement is 0.7 IU/ID dose and in Philippines it is 0.5 IU/ID dose.
97.How is RIG life saving?
• Administration of Anti-Rabies vaccine stimulates production of neutralizing antibodies by the patient`s immune system. Protective levels of antibodies (of more than 0.5 IU/ml of serum) are seen 7 to 14 days after the initial dose of vaccine (window period). Moreover when the bites are on the head, neck, face & hands, the incubation period will be shorter.
Thus the patients are vulnerable to develop rabies during this window period of 7 to 14 days. RIGs are readymade anti-rabies antibodies and provide passive immunity to rabies.
98.What are the situations where RIG is indicated?
• Following situations need RIG:-
1. All Category III exposures.
2. Bites by all wild animals viz. by mongoose, jackal, fox etc.
3. Even Category II exposures in immuno-compromised/immunosuppressed individuals including HIV infected people & AIDS patients.
4. RIGs should also be administered in Category III exposures even by vaccinated pet animals.
5. RIGs can be used in pregnant women and lactating mothers.
99.What are the limitations of Polyclonal Serum (RIG)?
• Difficulties associated with the use of Polyclonal Serum (RIG) samples might consist of:-
1. Batch-to-batch variation.
2. Risk related to the use of human blood products. Because HRIG preparations are of human origin, they need to be treated to minimize the risk of transmission of infectious agents.
3. Difficulties in finding immune donors during sudden mass exposures might lead to a low availability of RIG.
4. Because of discontinued ERIG production by international manufacturers, supply of ERIG relies on regional production.
5. The use of ERIG has raised ethical issues and has been condemned by animal protection groups.
100.What are the types of RIGs?
• There are two types of RIGs:-
(1) Human RIG (HRIG): Available as 2 ml. vial with a potency of 150 IU/ml.
(2) Equine RIG (ERIG): Available as 5 ml. vial with a potency of 300 IU/ml.
(TO BE CONTINUED—-)