FOREIGN BODY SYNDROME (FBS) IN CATTLE & BUFFALOES :DIAGNOSIS, TREATMENT ,PREVENTION & MANAGEMENT
Cattle are indiscriminate feeders. They can’t differentiate between metallic materials in feed and do not completely masticate the food before swallowing (Aiello et al., 2016). Hence, they ingest sharp metallic objects like nails or wires. These foreign bodies get settled in the reticulum, due to its honey comb structure. The foreign bodies remain there and starts puncturing the reticulum causing a disease which is known as “Foreign body syndrome” which is also known as “Hardware Disease”, “sharp foreign body syndrome” (SFBS) or “traumatic reticuloperitonitis” (TRP). Foreign body syndrome has been recorded in bovine specifically in developing countries due to lack of recycling industrial wastes (Vanitha et al., 2010) and due to improper waste management. The disease is commonly reported when green chop, silage and hay are made from fields that have old rusting fences or baling wire or when the grazing pastures are in the areas where buildings have recently been constructed. Malnutrition and unbalanced dietary habits can also lead to ingestion of materials other than normal food including wastes (Otsyina et al., 2015) leading to foreign body syndrome. The disease has economically a great impact as it causes severe reduction of milk and death of animals. The magnitude of loss to dairy industry can be assessed from the fact that this complex has been responsible for more than 15 % of all the natural deaths in dairy and beef animals (Sharma et al., 2015). In India, it has a high prevalence ranging from 23% to 87% (Hussain et al., 2018). Economic losses and the number of animals affected are so high that it has driven researchers to go deep in the diagnosis and treatment of this syndrome (Makhdoomi et al., 2018). There is a great variability in clinical signs of foreign body syndrome. Therefore, it is a significant challenge to diagnose and manage FBS. Definitive diagnosis is a necessary prerequisite to decide on the surgical intervention to be employed. It is a clinical demand to evolve strategies to diagnose and manage traumatic reticuloperitonitis at an early stage (Rajput et al., 2018). Haematobiology and biochemistry plays an important role in the diagnosis and to know the extent of damage due to foreign body syndrome.
Foreign body syndrome is usually associated. with changes in hemogram of the affected animals which are very useful indicator for the diagnosis in buffaloes (Kaur and Singh, 1994). Complete blood count (CBC) in suspected cases is of very high value in diagnosis and prognosis (Chander et al., 1997). Total leucocytic count (TLC) and differential leucocytic count (DLC) not only help in diagnosis of clinicopathological conditions but also provide enough indication about their prognosis (Kaur and Singh, 1994). Ingestion of indigestible foreign materials by cattle and buffaloes is a common problem worldwide, known as foreign body syndrome (FBS) (Aref and Abdel-Hakiem, 2013). This syndrome is more common in bovine than in small ruminants because they do not use their lips for prehension and are more likely to eat chopped feed (Misk and Semieka, 2001; Ashfaq et al., 2015). Moreover, indiscriminate feeding habits, feed scarcity, industrialization and mechanization of agriculture are predisposing factors for FBS (Semieka, 2010). The non-metallic foreign body syndrome is a silent killer disease resulting from ingestion of polywastes, rubber, plastics, leather materials, ropes, clothes and cement bags (Reddy and Sasikala, 2012). The presence of foreign bodies in the rumen and reticulum hampers the absorption of volatile fatty acids, consequently leading to reduction in the rate of animal fattening (Igbokwe et al., 2003). When a metallic object such as a wire or nail is swallowed and punctures the reticular wall, the condition is known as “Hardware Disease”, “sharp foreign body syndrome” (SFBS) or “traumatic reticuloperitonitis” (TRP). Several complications result from ingestion of indigestible foreign materials such as chellitis, gingivitis, glossitis, stomatitis, pharyngitis, tonsillitis, chock, esophagitis, rumenitis, ruminal impaction, acute or recurrent rumen tympany, localized or diffuse reticuloperitonitis, reticular adhesion and diaphragmatic hernia. Other consequences include pericarditis, reticular fistulation, reticular, diaphragmatic, mediastinal, hepatic, splenic, lateral and ventral abdominal wall abscesses, vagal indigestion, rupture of left gastro-epiploic artery, traumatic pneumonia and pleurisy (Roth and King, 1991; Floeck and Baumgartner, 2001; Abu-Seida and AlAbbadi, 2014). These complications depend mainly upon the nature, length and direction of penetration of the swallowed foreign body (Abouelnasr et al., 2012). This condition produces devastating economic losses due to severe reduction in milk and meat production, treatment costs, potential fatalities and fetal losses in affected pregnant animals (Nugusu et al., 2013). It may prove lethal because the bacteria and protozoa can contaminate the body cavity resulting in peritonitis and the heart and diaphragm may be punctured by the ingested object, causing their failure (Abu-Seida and Al-Abbadi, 2015). Due to its high economic importance in dairy animals, FBS is still a matter of concern worldwide.
Foreign body syndrome in cattle is one of the common disorders in large ruminants reared in industrial areas. This includes three different conditions of surgical interest including traumatic reticulitis, traumatic reticuloperitonitis and traumatic pericarditis. The common etiology for these three conditions is ingestion of metallic foreign bodies through the feed material. During earlier days, this condition was diagnosed only on postmortem in cattle and buffaloes and was receiving less attention when compared to the infectious and contagious diseases (Aghion, 1953). Nowadays, due to urbanization and lack of adequate floor space, many of the animals are let outside for grazing, which make them eat the waste materials even. Besides this, depraved appetite due to mineral deficiencies also makes them consume non-sensical stuffs containing metallic objects leading to foreign body syndrome. With the advent of modern diagnostic equipment and increased cost of farm animals, many cases are now presented for the expert treatment, although after worsening of the disorder. Yet, there is only limited scope to succeed in treating the cases of traumatic reticulo-peritonitis and pericarditis.
Etiology:
The mode of animal prehension, indiscriminate feeding habits, bad nutritional management, heavy industrialization and human habitations are major predisposing factors for the occurrence of such condition in bovine (Khan et al., 1999). In addition, pregnancy, tenesmus and vigorous reticular contraction increase the potential for development of SFBS in bovine. Therefore, numerous animals have ruminal and reticular foreign bodies without development of clinical signs and sometimes these foreign bodies pass into feces. Mostly, the ingested foreign objects are lodged in the reticulum without harm due to their fixation by the honeycomb like reticular cells. The typical foreign body is a metallic object longer than 2.5 cm.
Diagnosis:
Without an accurate case history and when the victim is admitted after several days of ingestion of a metal object, the diagnosis is more difficult (Ramin et al., 2011). Differential diagnosis of SFBS is considered a challenge since the diseased animals show signs similar to those of several other diseases. Uterine or vaginal trauma, metritis, perforating abomasal ulcers, grain overload, ketosis, abomasal displacement, hepatic abscesses, pyelonephritis, intestinal adhesions to the abdominal wall and volvulus, should be considered during the differential diagnosis of SFBS. Thus the diagnosis should be based on case history, clinical symptoms, clinical examination, laboratory diagnosis, electrocardiography, radiography, ultrasonography, necropsy and histopathological diagnosis.
Clinical symptoms:
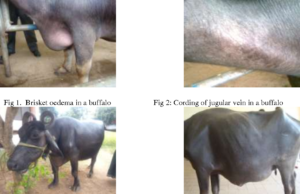
The SFBS is clinically characterized by severe depression, reluctance to move, abduction of fore limbs, progressive weakness (Fig. 2a), sharp drop in milk yield, variable appetite to complete inappetency, mild fever, ruminal stasis, recurrent tympany, scanty hard feces, abdominal pain and death (Singh et al., 2008). Sometimes, the diseased buffaloes show systemic reactions, jugular veins engorgement, brisket edema, abnormal lung and heart sounds, respiratory distress and regurgitation of food (Mostafa et al., 2015). Rarely localized abscess or fistula at the craniolateral and cranioventral abdomen is recorded (Figs. 2b and c). Animals with large amount of blunt foreign bodies show anorexia, depression, intermittent respiratory distress, recurrent rumen tympany, rumen stasis, dehydration, reduced milk yield, distended left para lumbar fossa and sometimes vomition (Reddy and Sasikala, 2012; Abu-Seida and Al-Abbadi, 2014). Clinical signs of traumatic pericarditis (TP) include tachycardia, muffled heart sounds, absence of lung sound in the ventral thorax, asynchronous abnormal heart sounds, distension of the jugular veins and pulsation, submandibular, brisket and ventral abdominal edema (Figs. 2d and e).
Clinical examination:
Tympanic sounds are heard on percussion with simultaneous auscultation of paralumbar fossa. By stethoscope, muffled heart beats, reduced gut sounds, and rapid breathing may be heard. Moreover, rectal palpation is a reliable method of diagnosing the rumen impaction in bovine and to exclude uterine or vaginal trauma and metritis (Reddy et al., 2014). Electronic metal detector can identify reticular metals but does not distinguish between perforating, non-perforating and nonmagnetic foreign bodies (Reddy and Sasikala, 2012). Briefly, pain tests include pinching of withers, walking on downhill and side stick method. Affected animals will not reflex ventrally when their withers are punched. They may also exhibit pain when a large bar placed under the animal’s sternum is forced upwards.
Laboratory diagnosis:
Laboratory tests may be helpful in diagnosis of FBS. Hemogram of the diseased animals shows anemia, increased packed cell volume and neutrophilia with a left shift (Reddy et al., 2014). Serum biochemical parameters of diseased animals show increased total protein, globulin, total bilirubin, ALT, ALP, P and decreased albumin/globulin ratio and Ca (Ghanem, 2010). Hyperproteinemia is noticed in buffaloes with acute and chronic local TRP, and reticular abscess. Hyper-beta-globulinemia is noticed in animals with chronic local TRP, reticular abscess and purulent pericarditis. Hyper-gamma-globulinemia is evident in animals with acute and chronic local TRP, reticular abscess and purulent pericarditis. Hypoproteinemia associated with severe hypoalbuminemia and very low A/G ratios characterizes animals with acute diffuse TRP, purulent and fibrinous pericarditis (Saleh et al., 2008) Animals with indigestible plastic materials in the rumen show mild hypocalcemia, hypophosphatemia, hypoglycemia and hypoproteinemia and increase in BUN, and increased values of Methylene Blue Reduction Test (MBRT), total volatile fatty acids and sedimentation activity test (Reddy et al., 2014). Severely affected animals may have coagulation abnormalities, as evidenced by prolonged prothrombin time, thrombin time, and activated partial thromboplastin time. Rumen liquor examination reveals a pH of 7.0-8.0 and nil or low protozoal motility and counts (Reddy and Sasikala, 2012). On hematological examination of animals with TP, erythrocytopenia, pronounced leukocytosis, with shift to left accompanied with neutrophilia, eosinopenia, monocytosis, lymphocytopenia, basopenia and anemia are usual. Hyperfibrinogenemia, hemoconcentration and increased serum AST, ALT, LDH, CPK and bilirubin are also reported. Moreover, cardiac troponin proteins have a value in determining the degree of heart damage (Gunes, 2008). Pericardiocentesis at the left 4th or 5th intercostal space may be applied under echocardiography to collect a sample of pericardial fluid. However, pneumothorax, fatal arrhythmia, pleuritis and cardiac puncture are potential complications. Protein concentration >3.5 g/dL and WBC count >2500/μL with straw yellow to slightly blood tinged, foamy, and foul smelling pericardial fluid are characteristics of TP (Elhanafy and French, 2012). The stay suture technique is inferior to other techniques due to high incidence of infection while Weingarth’s technique is superior to other techniques (Dehghani and Ghadrdani, 1995). In the skin suture fixation technique, the rumen is sutured to the skin using a continuous Connell suture pattern to invert the skin edges under the rumen to minimize contamination. In the stay suture technique, four stay stitches at the cranial, caudal, dorsal and ventral parts of the incision are performed to fix the rumen to the skin. Fixation of the rumen to the skin dorsally and ventrally and fixation of the ruminal incision to the skin incision cranially and caudally are applied by 6-8 towel clamps in the skin clamp technique. An aluminum ring with a rubber ring attached to its inner circumference is used during rumenotomy ring technique to fix the rumen to this rubber ring. In Weingarth’s technique, a Weingarth’s frame is fixed to the dorsal commissure of the incision by its thumb screw following laparotomy. Then the ruminal incision is fixed to the frame by multiple hooks. The Gabel rumen retractor depends mainly upon a device having a central hole that the rumen is pulled through. The rumen is fixed to the board by a series of bolts (Dehghani and Ghadrdani, 1995). Post-operative complications such as suture abscess, wound dehiscence, subcutaneous emphysema and local peritonitis at the surgical site are recorded (Nugusu et al., 2013). Reticular abscesses may be drained through an ultrasound-guided transcutaneous paracentesis or via rumenotomy into the rumen. Treatment of TP is often unrewarding and usually is addressed toward salvage or short term survival to calving. Diuretics are effective in eliminating the severity of peripheral edema, reducing venous return and preload in animals with pericarditis (Buczinski et al., 2010). Rarely, medical therapy with systemic antibiotics and drainage of pericardial sac permanently cures affected animals. Following thoracotomy, partial pericardiotomy or pericardiectomy is done in valuable animals. Pericardiotomy involves incising the pericardium, draining the fluid, removal of foreign body if present and thorough irrigation of the pericardial cavity with sterile isotonic saline solution containing antibiotics. An indewelling pericardial drain to allow twice-daily lavage, drainage and instillation of antibiotics may be conducted. Owing to high treatment cost together with poor results and high risk, under normal circumstances, animals with traumatic pericarditis should be humanely and timely euthanized (Ducharme et al., 1992).
Electrocardiography
The electrocardiography (ECG) is an important parameter for an animal with cardiovascular disorders (Reddy et al., 2015). Decreased amplitude of the QRS complex, electrical alternation (confguration of the P, QRS or T complexes regularly) and distortion or elevation in the ST segment are common ECG changes in cases of traumatic pericarditis (Foos, 1985; Tharwat, 2011).
Radiography
Laterolateral radiographic images are obtained from the thorax and the caudoventral reticulum with the animal standing, but for accurate localization of a foreign body, a dorsoventral view is necessary, which cannot be performed in adult cattle due to the great depth of the thorax (Braun, 2009). Furthermore, right or left lateral radiographs of the cardiac and reticular area of the decubitus animals are also reported (Athar et al., 2012). In the left lateral decubitus, it is possible to notice the cardiac silhouette and the diaphragm outline and obscured, showing opaque areas (Misk and Semieka, 2001). Despite, to avoid complications such as the spread of infections in the afected animals, it is advisable to perform the examination standing (Abu-seida and Al-abbadi, 2016). Radiographic changes may not be detected in early pericarditis and if there is a concomitant large amount of pleural fuid, it is not possible to diferentiate it from pleuritis (Imran et al., 2011; Athar et al., 2012). Radiography shows loss of thorax details, changes in heart shape and opacity, and radiopaque foreign bodies, such as needles and other metallic objects, can be observed, perforating the reticle, diaphragm or heart (Khalphallah et al., 2017; Sasikala et al., 2018). However, the non-visualization of the object may occur due to infammatory reactions and the presence of fbrinous exudates in the pericardial sac, not excluding the suspicion of the disease (Makhdoomi et al., 2018; Sasikala et al., 2018). In a study with cattle diagnosed with traumatic reticulopericarditis, 71% (20 of 28 animals) presented in the radiographic evaluation the obscured cardiophrenic angle and complete loss of the cardiac silhouette and ventral diaphragm due to extensive fbrinopurulent lesions (Braun et al., 2007b).
Ultrasonography
Ultrasonography is often chosen for diagnosis, imaging and characterization of efusions (Braun, 2009; Athar et al., 2012). Abdominal fndings reveal changes typical of traumatic reticulitis, such as waviness and thickening of the reticular wall, decreased motility and amplitude of reticular contraction and increased distance between the abdominal wall and the reticulum (Abu-seida and Al-abbadi, 2016). The presence of pleural fuid displacing the lungs and moderate to severe peritoneal fuid is evident in cattle and bufalo. (Braun, 2009; Kumar et al., 2012; Sagwan et al., 2018). Braun (2009) and Khalphallah et al. (2017) also verifed reticular displacement. There are often moderate to severe ascites attributable to heart failure (Athar et al., 2012). It is possible to notice the presence of reticular abscesses, which have an echogenic capsule of varying thickness involving a hypoechogenic to moderately echogenic center, in addition to abdominal and pulmonary abscesses that can have diferent sizes (2 to 20 cm) (Abuseida and Al-abbadi, 2016). Foreign bodies are observed by ultrasound as hyperechogenic structures that penetrate the reticular wall with comet tail artifact (Abu-seida and Al-abbadi, 2016). However, this examination hardly identifes metallic objects, such as magnets (Khalphallah et al., 2015).
Echocardiography
The echocardiographic examination is a simple and well-established tool for cardiac evaluation, being performed from the third to ffth intercostal space of both antimers (Buczinski, 2009; Hassan and Torad, 2015). In suppurative pericarditis, a large amount of hypoechogenic to echogenic fuid is usually observed in the pericardial sac, while in fbrinous is possible to evidence echogenic fbrin deposits and cords in the epicardium (Abu-seida and Alabbadi, 2016). Other fndings include cardiomegaly, thickening of the walls and increased cardiac contractions, in addition to vegetation of the tricuspid, mitral and pulmonary valves (Ghanem, 2010; Khalphallah et al., 2017) and the obscured heart due to the efusion (Schweizer et al., 2003).
Ferroscopy
Performing a metal detector scan on the ventral and lateral thoracic and abdominal wall can provide information on the presence of ferromagnetic foreign bodies (Sawandkar et al., 2009), although it is not possible to diferentiate between perforating and non-perforating objects (Reddy and Sasikala, 2012). In a study of 38 animals diagnosed with traumatic reticulopericarditis, 21 of them had foreign body detection through ferroscopy (Hussain et al., 2018), despite not being a tool widely used in suspected cases.
Pericardiocentesis
Pericardial fuid can be collected by centesis in the location with the greatest audibility of cardiac sound, usually in the fourth or ffth intercostal space on the left side (Athar et al., 2012). However, the procedure can cause deleterious efects, spreading the infection to the pleural cavity (Braun, 2009). In the chronicity of the disease, few amounts of liquid can be found, making it difcult to obtain a sample.
Cardiac biomarkers
Cardiac biomarkers are considered useful indexes for the early diagnosis of traumatic pericarditis. Cardiac Troponin I (cTnI), cardiac Troponin T (cTnT), creatine kinase myocardial band (CK-MB) and nitric oxide are considered important biomarkers of cardiac diseases because they elevate their serum concentration even without the characteristic signs of the disease (Mellanby et al., 2007; Neamat-allah, 2015; Attia, 2016). Venkatesan et al. (2020) found that the evaluation of cTnI at the place of care proved to be a simple diagnostic measure, which facilitated the assessment of myocardial involvement and cell damage in cattle afected by traumatic reticulopericarditis.
Laparoscopy
It is a promising technique for the diagnosis and treatment of several abdominal diseases in cattle (Babkine et al., 2006), because it provides greater practicality, rapid postoperative recovery and low risk of complications (Babkine and Desrochers, 2005; Seeger et al., 2006). Althought, massive adhesions hinder the visibility of the reticulum (Braun et al., 2020).
Necropsy and histopathological
In acute cases, distension of the pericardial sac is found foul-smelling liquid with fbrin and the appearance of ‘scrambled eggs’ (Abu-seida and Al-abbadi, 2016). In chronic cases, the pericardial sac is adhered to the pericardium by fbrinous junctions and thickened (Athar et al., 2012). Findings may also include presence of fuid peritoneal fuid with the presence of fbrin, fbrinous adhesions between the reticulum and / or diaphragm, spleen, abomasum, rumen, liver, abdominal wall and between the intestinal loops and omentum, as well as splenic, pulmonary abscesses, reticular, abdominal and hepatic, depending on the direction of the object when penetrating the reticulum (Ghanem, 2010; Chanie and Tesfaye, 2012; Abu-seida and Al-abbadi, 2016). The foreign body is usually found in the exam, although its recovery may not be possible even with its visualization in complementary exams, due to adhesions and extension of the infammatory process (Braun et al., 2007a). Histopathology shows the presence of nodular hyperplasia of the stratifed squamous epithelium in the reticulum (Sasikala et al., 2018), high infltration of infammatory cells in the pericardium and myocardium, mainly of neutrophils and mononuclear cells, in addition to myocardial hyalinosis (Ghanem, 2010; Abu-seida and Al-abbadi, 2016).
Treatment:
Mostly, conservative treatment is indicated in acute cases of SFBS. However, in chronic cases and late pregnant animals, conservative treatment is unsuccessful. Conservative treatments of SFBS include; external massage of the xiphoid process, oral administration of purgative, elevation of the fore limbs, fasting, supportive therapy, intra peritoneal antibiotic injection, and administration of a cage magnet (Horney and Wallace, 1984). However, it is unlikely that the magnet will move into the reticulum due to ruminal stasis. Rumen inoculation with 4–8 L of ruminal fluid from a healthy animal is beneficial in animals with prolonged ruminal stasis. If the conservative treatments fail to improve the animal after 3 days, rumenotomy is indicated. Rumenotomy is a rapid and successful procedure for diagnosis and treatment of FBS. The direct approach to the reticulum through a mid-line incision just posterior to the xiphoid is not preferable due to the possibility of diffuse peritonitis (Dehghani and Ghadrdani, 1995). However, a standing laparotomy is desirable due to the difficult manipulation of such large organ. The site of laparorumenotomy incision, size of the animal, and length of the surgeon’s arm should be considered to perform successful rumenotomy (Horney and Wallace, 1984). Moreover, gentle manipulation is recommended to minimize the spreading of infection in animals with traumatic reticulitis. Usually, there is little or no benefit from breaking down chronic adhesion because it tends to reform very rapidly. Also breakdown of recent adhesion is not advised because it may mask and surround an abscess (Weaver et al., 2005). Several techniques for laparorumenotomy including; skin suture fixation, stay suture technique, skin clamp technique, rumenotomy ring, Weingarth’s technique and the Gabel rumen retractor (rumen board) have been applied. All techniques are conducted through an approach in the left paralumbar fossa for successful access, exteriorization and securing of the rumen and minimizing contamination. The main difference between these techniques is the method by which the rumen is secured to the abdominal wall or skin (Niehaus, 2008)
Prevention:
It is difficult to prevent FBS in cattle and buffaloes, however certain precautions have decreased the incidence. These precautions include removal of ferrous and other potentially hazardous objects from field and lane edges, good nourishment and management of the animals, passing of processed foods over magnets to remove metallic objects, avoiding the use of baling wire, completely removing old buildings and fences, keeping animals away from sites of new construction, avoiding the pollution of grazing lands with plastic bags, hair, hoof, wool and avoiding the unsupervised grazing of animals (Nugusu et al., 2013; Reddy et al., 2014). Moreover, administration of reticular magnets at the age of 1.5-2years has become a popular preventive measure for hardware disease (Weaver et al., 2005). After oral administration, most magnets drop firstly into the rumen then move to the reticulum by ruminoreticular contractions. Metallic foreign bodies are attracted and fixed to the magnets, and consequently do not penetrate the reticulum as easily as when they are free. The extensive prophylactic use of these has reduced the incidence of TRP by 90-98% in cattle and 89-91% in buffaloes. A time dependent increase in the proportion of buffaloes developing TRP is noticed after 4 years of magnet administration, not due to loss of magnetic power but due to fixation of numerous foreign objects on the magnet. Therefore, reapplication of a second new magnet is recommended after four years of the first one particularly in animals at high risk (Al-Abbadi et al., 2014).
EDITED & COMPILED BY-DR S. MANJUNATH,TANUVAS
IMAGE-CREDIT-GOOGLE
REFERENCE-ON REQUEST