Genetically Modified Organisms (GMOs): A Complete Overview
Dr Dhara Gor, Dr Ashok Chaudhary, Dr Mayank Darji
Department of Animal Genetics & Breeding, College of Veterinary Science and Animal Husbandry, Kamdhenu University, Dantiwada, Gujarat
Corresponding Authors: drak97vet@gmail.com
- Abstract
An organism, except humans, carrying an altered genetic material that does not occur naturally through natural selection or mating are called as genetically modified organisms (GMOs).The most common method for producing GM animals is to inject the foreign gene into fertilised eggs through a process known as ‘microinjection’. Many other methods like Embryonic stem cells modification, Spermatocyte mediated gene transfer, Cloning: somatic cell nuclear transfer (SCNT), and Transposon-mediated gene silencing are used for GMOs production.
- Introduction
Genetically modified organisms (GMOs) are defined as any organism, except humans, carrying an altered genetic material that does not occur naturally through natural selection or mating and including techniques of recombinant DNA (rDNA) technology along with microinjection techniques as a tool for genetic modifications. All such tinkered organisms are known by synonymous terms like “genetically engineered,” “genetically modified,” “transgenic,” “biologically engineered,” and “biologically modified” organisms.
A genetically modified organisms (GMOs) is an organism whose genetic material has been altered using genetic engineering techniques. This involves manipulating an organism’s DNA, the hereditary material that contains the instructions for the organism’s growth, development, and functioning. GMOs can be plants, animals, microorganisms, or other life forms that have had specific genes from other species inserted into their DNA to achieve desired traits. The process of creating GMOs involves identifying a gene with a desired characteristic, isolating that gene, and then inserting it into the target organism’s genome. This can result in organisms with improved traits such as increased resistance to pests, enhanced nutritional content, faster growth rates, or tolerance to environmental conditions like drought. GMOs have been used in various fields, including agriculture, medicine, and research.
- Genetically Modified (GM) animal
An animal whose genome has been transformed or modified through recombinant DNA technology is referred to as a Genetically Modified (GM) animal. The first GM cattle were reported in 1985 and since then biotechnology developments have allowed researchers to engineer additional GM animals for agricultural and medical purposes. GM cattle have been engineered to improve important traits, such as growth rate, meat quality and quantity, milk production, survival, and disease resistance. Pigs have been modified to grow faster and produce more meat while consuming less feed. Experts have paid special attention to piglets with a focus on improving their health, prolonging their survival, reducing the rate of infectious diseases, and strengthening their immune systems. Sheep have been engineered to improve wool production and resist viral or bacterial infections. The growth rate of chickens and the disease resistance and survival of chicks has also been amplified through engineering. GM cows and buffaloes with bovine serum encephalopathy (BSE) resistance have also been bred successfully. Moreover, fish have become a target for genetic alterations (i.e., salmon, tilapia, and carp species) with a special focus on increasing the quantity and quality of their meat. GM fish have been sold at pet shops; however, most transgenic animals are still a long way from commercialization due to biosafety and risk assessment issues.
- How are GM animals created?
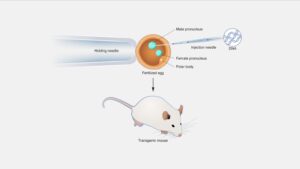
The most common method for producing GM animals is to inject the foreign gene into fertilised eggs through a process known as ‘microinjection’. For mammals, the injected eggs are placed into a ‘foster’ mother where they develop to term. If the foreign gene has been successfully incorporated into the egg’s original DNA, the resultant offspring will carry the extra, foreign DNA. When this GM animal mates and produces offspring, the foreign gene is inherited in the same way as normal DNA. In this way, scientists can breed a line of GM animals that carries the extra DNA. A diagrammatic representation of the technique is provided in Diagram 1 below.
- Methods for Introduction of Transgenesis
The introduction of transgenesis can be roughly grouped into three categories based on the changes in the genome desired: the introduction of a new gene, the replacement of a gene through homologous recombination, and the alteration of gene expression through epigenetics. Before venturing into the details of the methods the foremost point of consideration is identification of the gene of interest. Identification of the gene of interest is the most vital spoke in the wheel of genetic engineering. When generating a GM organism, a transgene is inserted into the host animal’s genome. Among the many available routes for screening and identification of the gene of interest, the exact approach would depend on the organism and the rDNA technology available for that organism, along with other factors like whether the mutation is recessive or dominant, which screens are available for identification of the mutant and the wild-type phenotype, known homology with other genes, etc. The gene of interest can be screened from sequence homology, targeting the RFLP (restriction fragment length polymorphism) markers around the gene of interest, or from the cDNA libraries (utilizing the technique of “primer walking”) available in the public domain or by creating your own libraries.
(a) Microinjection
Microinjection or pronuclear injection was at one time the most successful method for producing GM animals. Around 400–500 copies of the transgene construct are directly injected using a very fine needle into one of the two pronuclei of the fertilized embryo. Transgene gets inserted into the host genome randomly with low efficiency and such in-vitro fertilized embryo thus generated is then cultured in the artificial environment and transplanted into an impregnated surrogate mother. Microinjection at a later stage (after the single celled stage) leads to the appearance of “mosaic” patterns. The microinjection technique has been used most widely for biopharming. This method has its own lacunae, too, like a very low degree of efficiency of ∼5%, high screening costs, and being unsuitable for creating GM birds/poultry as it is challenging to access the fertilized egg at the single-celled stage. Therefore, the method of viral transfection is best suited in case of aves. Nonetheless, microinjecting the gene construct into the zygote was reported to produce transgenic birds with some success. Advances in combining pronuclear injection with integrases and recombinases hold greater possibilities for creating targeted mutagenesis. The first report of generating transgenic mice with high muscle mass through direct microinjection of the recombinant lentiviral vector containing shRNA against the myostatin gene holds promise for quality food from livestock.
(b) Viral Transfection
Lentiviruses—slow growing members of the viral family of retroviridae categorized by long incubation periods—have been the most utilized vectors to deliver the transgene by virtue of their ability to integrate with the host genomic DNA and proliferate with every replication of the host genomic content. However, adenoviruses—the nonenveloped double stranded DNA viruses of the family adenoviridae—are also being used. Recombinant adenoviruses have been used in swine to generate vaccines against swine influenza virus. The “replication competent” viruses reintegrate the transgene multiple times and ensure transgene overexpression in the offspring and sometimes even in other animals coming into contact, due to their high infection rates. In contrast, “defective competent” viruses can transfect the cells only once. Retrovirus-mediated transfer has been successfully used to transfer a foreign gene into cows, chickens, monkeys, swine, and sheep through mammalian and avian retroviruses like Rous sarcoma virus (RSV), Moloney leukemia virus (MLV), Avian leukosis virus (ALV), Simian virus, etc. Viral mediated genetic modification has some major practical limitations:
- It limits the size of the transgene to be inserted, making it unviable for larger gene constructs.
- The long terminal repeats of the virus interfere with the promoter of the transgene.
- Viral vectors lack the ability to replicate in early embryo cells, resulting in “chimeras” where the transgene is expressed in only some of the cells of the GM animal.
(c) Embryonic Stem Cells Modification
Embryonic stem (ES) cells are self-renewing pluripotent cells derived from the inner cell mass of early dividing embryos called blastocysts. The first successful establishment of the embryonic stem cell culture was carried out from a murine embryo in the early 1980s and concerted the effort for the isolation of ES cells from nonhuman primates and finally from humans. The transgene delivery system, as in the viral delivery system, could be “directed” or “random,” depending on the strategy implied. Directed gene “knock-in” or “knock-out” relies on homologous recombination, a DNA repair mechanism where the nucleotide sequences are exchanged between genomic and exogenous sequences through the event of crossing over between homologous sequences. The transformed ES cells are then transferred into a recipient embryo, implanted into an impregnated surrogate, and “chimeras” or “completely transformed” animals are screened.
(d) Spermatocyte-Mediated Gene Transfer
Transgene is incorporated into the spermatocyte through electroporation, polyethylenimine-mediated gene transfection, or viral-mediated delivery. The transfected spermatocyte is used as a vector after in-vitro fertilization, where the attachment of exogenous DNA to the sperm is facilitated by the specific DNA binding proteins (DBPs) present on the postacrosomal surface of the sperm. The first transgenic mice were generated through sperm-mediated gene transfer, mixing plasmid DNA with spermatocytes. But the results of sperm-mediated gene transfers are marred by low repeatability and efficiency in the case of mammals. Intra-cytoplasmic sperm injection directly into unfertilized oocytes has substantially increased the efficiency of transgene expression in the recovered offspring.
(e) Cloning: Somatic Cell Nuclear Transfer (SCNT)
The emergence of cloning technologies facilitating nuclei transfer from the somatic cells brought a paradigm shift in the biological science in generating transgenic livestock. In somatic cell nuclear transfer, enucleation of oocytes is followed by the fusion of the donor cell and activation of this reorganized embryo. SCNT has been efficacious in many animal species, including those reared for domestic, wildlife, or laboratory research. It has been the method of choice for generating larger transgenic animals, beginning with the cloning of Dolly (the first cloned animal) and later carried out in other animals, producing transgenics with greater muscle mass and therapeutic applications. It is a potent tool for the extension of superior breeding stock of farm animals for food.
(f) Transposon-Mediated Gene Silencing
Transgenesis can be induced in livestock through transposons. Transposons, or “jumping genes,” are the unique DNA elements that traffic around the genome. This inherent property of these elements can be harnessed to induce genetic modification in genome generating transgenic livestock. With the discovery of sleeping beauty, PiggyBac- and Tol2-like transposons, interest have been reinvigorated in this domain. Successful generation of transgenic swine has been achieved through the introduction of double stranded RNAi (ribonucleic acid mediated interference) cassette silencing the cyctic fibrosis transmembrane conductance regulator (CFTR) through the introduction of transposons (Sleeping Beauty/Tol2/ PiggyBac, etc.) flanking the nucleic acid construct containing the transcriptional unit on both sides. Transgenic rabbits and pigs carrying a fluorescent reporter have also been generated using a similar approach.
- Advent of GMOs
A scientific breakthrough was achieved when the first recombinant mouse was generated through microinjection into the pronuclei of mouse oocyte. Since then, genetically modified (GM) mice have been an indispensable part of medical research, serving as disease models for various congenital, metabolic disease, cancer, and aging disorders. Other animals have also been genetically modified for reasons other than research. Considerable success has been achieved in the introduction of novel traits into different animals. There is now an extended list of cows, swine, sheep, and goats genetically modified or fortified for food (and other applications) generated through various rDNA technologies and epigenetic approaches, creating heritable and nonheritable changes in the genome.
Transgenesis is an extension of the conventional breeding practices:
- Being more robust, direct in approach, and effective than conventional breeding;
- Bringing about heritable changes once a foreign gene is inserted;
- Not restricted by fertility barriers in which only closely related species could be crossed, even heterologous genes have been inserted into animals with substantial success, for example, the Enviropig™, containing a phytase-producing gene from Escherichia coli; and
- Inducing both heritable and nonheritable changes with the usage of appropriate methodology.
What are the potential hazards associated with GM animals?
Possible hazards of developing GM animals include:
- Novel or increased allergic reactions and toxic effects if they are eaten,
- Changes in the behavior of the GM animals such as increased aggression,
- Changes in the ability of the GM animal to act as a human disease reservoir,
- Impact on the ecosystem if the GM animals escaped or are released into the environment.
The likelihood of these happening is generally considered to be relatively low but certainly should not be neglected.
- Is it ethical?
For some, genetic modification of animals is unethical because they feel that it is a form of disrespect to animals or is a violation of animal rights. However, many others believe that an acceptable balance exists between the need to minimize animal suffering and the need to maximize gain to medicine, agriculture, and scientific understanding. Traditional breeding is also a form of genetic manipulation.