Goatpox: An Egregious Disease of Goats
Gnanavel Venkatesan, Sabarinath T, Amit Kumar, Aditya Sahoo, Ajayta Rialch and V Bhanuprakash
Introduction:
Goatpox, which has been reported from all parts of the world, is a malignant disease of goats characterized by fever and generalized pocks. Even though strains of this virus show a host preference, serologic evidence, cross infection, and cross-protection experiments indicate that goatpox virus (GTPV), which is otherwise indistinguishable from sheeppox virus (GTPV), should be considered as causing a single disease complex, must be named as capripoxvirus. Goatpox results in overall morbidity and mortality upto 73% and 57.6%, respectively. Although, the disease is generally benign in adults, recent outbreaks revealed almost 100% morbidity and mortality in kids.
Virus Characteristics:
GTPV is an elongated and brick-shaped complex symmetry virus and is morphologically similar to sheeppox virus. Electron microscopic size of GTPV is ~250-280×212-260 nm. The Iranian and Egyptian strains of GTPV are more resistant than Dushanbe strain as heating at 560C for l hour did not drop their titres significantly. However, GTPV adapted in sheep testis and sheep embryo dermis cell culture could be inactivated in 45 min and 30 min at 500C and 560C, respectively. Several researchers found GTPV to be sensitive to ether and chloroform treatment. However, some ether-resistant strains of GTPV have been reported. GTPV has also been inactivated using formalin. However, trypsin and EDTA failed to completely inactivate GTPV after 2hours of treatment. Replication of GTPV was inhibited by actinomycin D and bromodeoxyuridine. GTPV can be lyophilized without any loss of infectivity. Different isolates of GTPV are indistinguishable by neutralization and cross-protection tests in goats suggesting antigenic stability. However, there is scientific evidence that isolates of GTPV are antigenically different since the Gorgan strain of GTPV revealed 100 times higher neutralization in homologous system than with heterologous system.
Epidemiology:
After the initial report of goatpox by Hansen in 1879 from Norway, the disease was observed in Macedonia during the first world war resulting in 5 to 15% mortality. The disease is endemic in Central and North Africa, the Middle East, Turkey, Pakistan, India, Nepal and China. The disease is comparatively benign in adult goats, but in kids it is generalized with high mortality rate. The morbidity ranged from 38 to 90% and mortality from 0 to 85%. Even though several researchers claim that goats are the only susceptible species for GTPV, there are reports of artificial transmission of GTPV in horses, dogs, poultry, monkeys, rabbits and sheep. The strains of GTPV isolated from Indian sub-continent and Middle East are host specific affecting goats only. However, a strain from Kenya was not host specific and caused outbreaks in mixed flocks affecting both sheep and goats. Thus, the term capripox was proposed irrespective of the host species involved.
Transmission:
The disease may be transmitted or disseminated from one area to another by direct or indirect contact or through flies. The fly Stomoxys calcitrans may transmit the capripox virus mechanically within 1 hour of ingesting the virus. Transmission is also possible by flies which have ingested the virus 24 hours previously as virus can persist upto 4 days. Experimentally the disease can be transmitted by subcutaneous inoculation of infected blood and spleen from infected goats and scab suspension into susceptible hosts. Its transmission by scarification of virus is also possible. Researchers observed more rapid transmission of disease from animals that had well-developed clinical signs than those which died of peracute disease or that had only mild clinical signs.
Clinical signs and Pathology
After an incubation period of 8 to 12 days, pyrexia (40-42°C) is observed which is followed by anorexia and skin lesions. The affected animals stand apart from the herd and are reluctant to move. Purulent or mucopurulent lacrimal discharge following conjunctivitis leading to corneal opacity and blindness have been observed (Fig. 1A). Severe mucopurulent nasal discharge and pneumonia leading to death are more common in kids whereas, adults developed diarrhoea syndrome. In pregnant animals, mastitis and abortion are observed. Eruptive skin lesions are observed in infected animals all over the body starting from tongue to the tip of tail including hairy and hairless parts like face, perineal region and flank, lips, muzzle, face, nose, eyelids, ear, abdomen, thorax, vagina, teats and udder and fore and hind limbs. In adult animals, the lesions were discrete and widely spaced on the udder, mouth commissures, shoulders and trunk, whereas, in young animals the lesions were more generalized covering most of the body parts. Pox lesions have been observed on mucous membrane of the alimentary tract from buccal mucosae to large intestines, lymph nodes, liver and respiratory tract including lungs. Lesions on lungs and liver ranged from numerous bright red and firm nodules of 2-6 mm diameter to greyish white foci depending on the stage of infection.
Diagnosis:
Pox diseases are usually identified according to their clinical signs and gross pathology and the host species affected. It appears that the host preference shown by these viruses with respect to either goat or sheep, accompanied by the case history, may be regarded as partially affirmative for either goat pox or sheep pox, but accurate identification requires laboratory studies. The non-infectious soluble antigen fraction alone can efficiently replace the use of infectious virus, which pose a considerable disease security problem as the virus is highly infectious. In addition, the diagnosis of goat pox by classical virological or serological techniques that depend on live viruses is not suitable in countries where the viruses are exotic and live viruses are not available. Other commonly used serological diagnostic tests for the detection of GTPV and positive sera samples are Agar gel precipitation test (AGID), Counter-immunoelectrophoresis test, Latex agglutination test, Reverse-phase passive hemagglutination test, Single radial hemolysis test and Enzyme-linked immunosorbent assay. Various methods of ELISA are available to diagnose goat pox, but problems such as a considerable background reaction and the requirement for special reagents such as recombinant proteins, often limit their use as routine screening tests. In recent past, recombinant proteins of GTPV/SPPV present (P32, A27L, A4L, A12L and A33R) at different layers of virus structure have been over-expressed in heterologous system and used in ELISA for detection of antibodies and this avoids the use of live virus antigen in diagnostic settings.
Polymerase chain reaction and real time PCR:
Although sensitive, methods such as ELISA and virus isolation in cell culture fail to detect virus particles that are bound to neutralizing antibody, and the sensitivity of precipitation and agglutination tests is relatively low. Hence, PCR now employed for the identification of GTPV in skin biopsies and cell culture. PCR-based diagnostic methods (Fig. 1B) are exceptionally effective for the diagnosis of goatpox in suspected skin samples obtained from the field. However, differential diagnosis of goat pox and sheep pox based solely on the PCR technique is not yet feasible, but may be possible by restriction analysis of PCR-amplified products targeting P32 gene of CaPV genome. For quantification of GTPV and SPPV in clinical samples, P32 and DNA Pol based real-time PCR are reported for sensitive and specific detection of the genome.
Loop Mediated Isothermal Amplification assay (LAMP):
The LAMP could produce a highly specific and sensitive reaction compared to PCR and could be performed at a single temperature using a simple heating block and can be developed as POCT (point of care testing) in resource limited field settings. LAMP assays targeting P32, Poly (A) polymerase, DNA polymerase genes of CaPV have been reported for rapid detection of GTPV/SPPV and also to differentiate them. DNA polymerase gene-based LAMP assays for simple and fast identification of CaPV by characteristic ladder-like pattern in AGE and visual detection using SYBR Green I dye (Fig. 2).
Prevention and Control:
Vaccines:
Inactivated Vaccine:
Formalized and aluminium hydroxide gel adsorbed vaccines were found to be effective against goatpox. This vaccine was superior to lyophilized BPL inactivated and adjuvanted vaccines as it controlled outbreak of disease. This vaccine provided immunity against homologous and heterologous challenges and elicited CMI and humoral immune responses This vaccine provided protective immunity against goatpox, sheeppox and contagious ecthyma for 6 months.
Live attenuated Vaccine:
Earlier, GTPV attenuated in lamb kidney, lamb testicular cell culture and goat kidney cell culture offered good immunity and produced only a mild local reaction at the site of inoculation in adult animals. However, it produced strong reaction in young animals. Virulent strain of GTPV grown in sheep kidney cells (at 56th passage level) immunized indigenous Iranian goats but the same virus caused 96% mortality in imported goats when given subcutaneously. A vaccine developed in Bangladesh by Kitching and co-workers was found to be effective, stable and safe both for sheep and goats throughout the capripox enzootic areas and the vaccinated animals were immune from 6 to 8 days upto a year or more. This vaccine was found to be safe for different breeds, ages and at any stage of gestation. The antigenicity of this vaccine virus was retained when it was lyophilized and stored at 40C. In India, a live vaccine attenuated in Vero cells at passage 60 was found safe, efficacious and potent in goats by laboratory and limited field trials and currently this vaccine is under commercialization process.
Treatment:
Sick animals should be kept in clean, well-ventilated pens and fed on high plain of nutrition along with terramycin @ 5mg/kg body weight l/M or oxytetracycline @10 ml/day J/V for 5 successive days to check bacterial infection. Recurrence of capripox lesions on the udder of pregnant goats may largely be resolved by topical application of Zovivax (5% acyclovir) cream, 5 times daily at 4-hour interval for 4 days. Eyes and nostrils should be washed with 2% boric acid and 1:10,000 potassium permanganate solution, respectively. Terramycin ointment and powder may be applied topically on scab or ulcerated lesions and oleum eucalyptus inhalation or coramine may be used as respiratory stimulant.
Selected references
- Balamurugan V, Jayappa KD, Hosamani M, Bhanuprakash V, Venkatesan G, Singh RK (2009) Comparative efficacy of conventional and TaqMan polymerase chain reaction assays in the detection of capripoxviruses from clinical samples. J Vet Diagn Invest 21:225–231
- Balinsky CA, Delhon G, Smoliga G, Prarat M, French RA, Geary SJ, Rock DL, Rodriguez LL (2008) Rapid preclinical detection of sheeppox virus by a real-time PCR assay. J Clin Microbiol 46:438–442
- Bhanuprakash V, Hosamani M, Singh RK (2011) Prospects of control and eradication of capripox from the Indian subcontinent: a perspective. Antiviral Res 91(3):225–232
- Bora DP, Venkatesan G, Bhanuprakash V, Balamurugan V, Prabhu M, Siva Sankar MS, Yogisharadhya R (2011) TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of Orf infection. J Virol Methods 178:249–252
- Garner MG, Sawarkar SD, Brett EK, Edwards JR, Kulkarni VB, Boyle DB, Singh SN (2000) The extent and economic impact of sheep pox and goat pox in the state of Maharashtra, India. Trop Anim Health Prod 32:205–223
- Heine HG, Stevens MP, Foord AJ, Boyle DB (1999) A capripox virus detection PCR and antibody ELISA based on the major antigen P32 the homolog of the vaccinia virus H3L gene. J Immunol Methods 227:187–196 Hosamani M, Mondal B, Tembhurne PA, Bandyopadhyay SK, Singh RK, Rasool TJ (2004a) Differentiation of sheep pox and goat poxviruses by sequence analysis and PCR-RFLP of P32 gene. Virus Genes 29:73–80
- Hosamani M, Nandi S, Mondal B, Singh RK, Rasool TJ, Bandyopadhyay SK (2004b) A Vero cellattenuated Goatpox virus provides protection against virulent virus challenge. Acta Virol 48 (1):15–21
- Madhavan A, Venkatesan G, Kumar A (2016) Capripoxviruses of small ruminants: current updates and future perspectives. Asian J Anim Vet Adv 11:757–770
- Rao TVS, Bandyopadhyay SK (2000) A comprehensive review of goat pox and sheep pox and their diagnosis. Anim Health Res Rev 1(2):127–136
- Venkatesan G, Balamurugan V, Bhanuprakash V, Singh RK, Pandey AB (2016) Loop-mediated isothermal amplification assay for rapid and sensitive detection of sheep pox and goat pox viruses in clinical samples. Mol Cell Probes 30(3):174–177
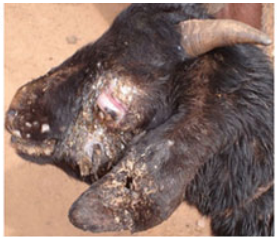
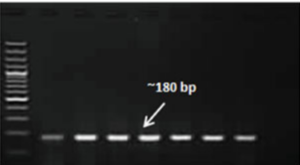
Fig. 1: Clinical picture of goatpox lesions at eye, ear and mouth regions (A) and DNA polymerase gene (180bp) based conventional PCR amplification of GTPV/SPPV in agarose gel analysis
Fig. 2. DNA polymerase gene-based LAMP assays for simple and rapid detection of CaPV gDNAs [Typical ladder-like pattern in agarose gel analysis (A) and SYBR green I dye-based visual detection (B) of CaPV DNAs (Lane 1: GTPV; Lane 2: SPPV; Lane 3: ORFV; Lane 4: NTC)

