HOW TO CHECK WATER ADULTERATION IN MILK BY USING LACTOMETER IN HOME
Now-a-days the milk adulteration is mostly detected using various chemical tests. These methods are tedious, time consuming and costly. Also the knowledge of the tests is necessary. The nutritional value of milk to human health needs no introduction; it also has traditional impact on Indian society. At the same time it is alarming that many vendors adulterating it with water, detergents, caustic soda, starch, formalin, urea, ammonium sulphate, sodium carbonate which have harmful effect on the human health. The greed for money has pushed them to the extent of producing synthetic milk which has no nutritional content. “Adulteration” is a legal term meaning that a milk product fails to meet federal or state standards. Adulteration is an addition of another substance to milk in order to increase the quantity of the milk in raw form or prepared form, which may result in the loss of actual quality of milk. Milk adulterated is mainly done for financial gain but it can also be adulterated due to unhygienic conditions such as processing, packaging, transportation, distribution etc. Water is the most common adulterant used which decreases nutritional value of milk and lowers the quality of milk.
There are a number of ways to check if the milk you are buying is fit for consumption or not. It is important that you carry out these checks periodically to ensure that there are no unwanted chemicals in the milk you buy.
What is milk adulteration / synthetic milk / artificial milk?
Synthetic milk is entirely a different component with a high degree of adulteration to increase the volume of milk and thereby the profits. From the natural milk received from cows and buffaloes, cream, butter and fat are separated using the cream separator machine. To make this cream-less milk again creamy, urea, detergent, caustic soda, starch oil, glucose, shampoo, hydrogen peroxide, etc are mixed, thus making it synthetic milk. A peculiar type of uneasiness pervades the people these days pertaining to the matter of synthetic/artificial milk. This milk has headed its journey from cities to villages now.
Harms caused by the use of synthetic milk
According to physicians, use of synthetics milk inflicts very serious harms on human body causing swelling in the eyes and complications in liver and kidney. Apart from this, synthetic milk proves deadly for pregnant women and patients suffering from conditions of heart ailment and high blood pressure. What is worse is that this synthetic milk is extremely poisonous for small children. Continuous use of the synthetic milk turns the human body into farm house of diseases. Synthetic milk is a slow poison which though does not kill at once, but it slowly makes the body a fertile ground for diseases.
Here are a few easy tests you can do at home:
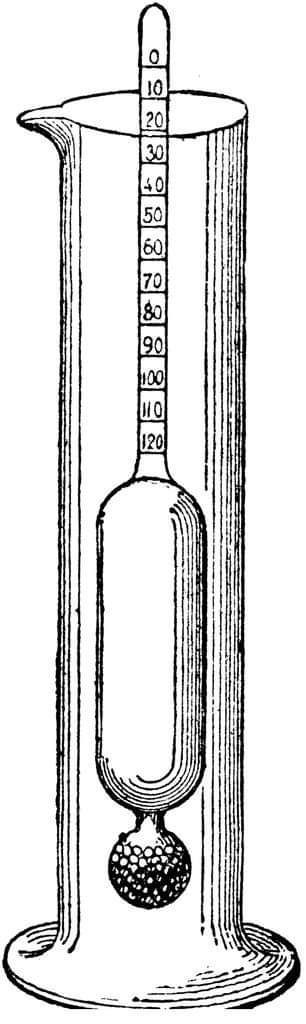
Lactometer is a cylindrical vessel made by blowing a glass tube. One side of glass tube looks like a bulb filled with mercury and another side is thin tube with scale. For milk testing, lactometer is dipped in milk which we are testing. In lactometer, the point up to which it sinks in the pure milk is marked after that put in water and marked at the point up to which it sinks in water. It sinks less in milk than water because as we know milk is denser than water. In the lactometer there are two portions i.e. ‘M ‘and ‘W’ which is divided in three parts and marked as 3, 2 and 1. That indicates the level of the purity in milk.
Here are some steps below mentioned for milk testing –
Step 1- Whenever you want to test the milk purity, you just put the lactometer in milk.
Step 2- If it sinks up to the mark ‘M’ which is mentioned in the lactometer that means milk is pure or if not that means milk is impure.
Step 3- If the milk is mixed in water then it would sink higher than the mark ‘M’.
Step 4- If it stands at the mark 3 that means milk is 75% pure and respectively 2 for 50% purity and 1 means 25% purity.
Testing Methodology
Preparation of milk sample for analysis
Warm the sample to 37- 40°C by transferring it to the beaker and keeping it in a water bath maintained at 40 – 45°C. Stir slowly for proper mixing homogenization. Mix sample thoroughly by pouring back into the bottle, mixing to dislodge any residual fat sticking to the sides and pour it back in the beaker. During mixing do not shake the bottle vigorously. Allow the sample to come to room temperature (26- 28°C) and withdraw immediately for analysis. If small clots or lumps are observed in the sample which cannot be dispersed, a few drops of liquor ammonia may be used during homogenization. If even after homogenization the sample shows lumps or clots or droplets of oil are visible suggestive of curdling /splitting of milk, the sample should be deemed unfit for analysis and rejected.
- Determination of Solids-Not-Fat (SNF) in Milk using lactometer
Measurement of specific gravity or density by a lactometer is based on the Archimedes principle. A floating object sinks till it has displaced a weight of fluid equal to its own weight. The greater the volume of displaced fluid, smaller is the density of the fluid and lower is the lactometer reading. The total solids and the SNF content of milk are related to its fat percentage and specific gravity by the Richmond’s formula. The specific gravity of normal whole milk is 1.029 to 1.032 while for skim milk it is 1.036.
Milk drawn from the udder contains a large volume of air bubbles and the milk fat undergoes a gradual solidification. Due to these factors a gradual contraction in the volume of milk takes place with a slow increase in specific gravity to a maximum (Racknagal phenomenon). The specific gravity of milk will, therefore, vary with the duration and temperature of storage. This variation may be overcome by ensuring that the fat is completely in the liquid state before the specific gravity reading is taken. This is achieved by pre-warming the milk.
2.1 Apparatus
Lactometer (ISI lactometer): The lactometer should have the following specification.
S No. Characteristic Dimensions/
Divisions/
Tolerances
1 Range covered by scale, specific gravity 1.020 to 1.035
2 Specific gravity equivalent for each subdivision 0.0005
3 Permissible error at any point ±0.0005
4 Number of sub-divisions on scale 30
5 Number of sub-divisions beyond nominal scale of the top graduation One or nil
6 Length in mm of stem above top graduation mark 20 ± 5
- Scale length in mm 41 ± 4
- Distance in mm below the lowest graduation mark, where the stem has to remain uniform in diameter, Min
10 - External diameter in mm of stem containing scale (approx.) 4.0
- External diameter in mm of bulb 22 ± 1
- Length in mm of uniform stem, Max 80
- Volume in ml below bottom graduation line:
not more than
not less than
37
31 - Length in mm of the bulb 105 ± 5
ii. Thermometer: A thermometer having the following characteristics is suitable:
a) Overall length 255 ± 10 mm
b) Scale length 105 ± 10 mm
c) Distance between lowest graduation 110 ± 5 mm
line and bottom bulb
d) Scale range 10 to 45°C
e) Graduation interval 0.5°C
f) Scale accuracy ± 0.2°C
iii. Lactometer Jars: Cylindrical vessels made of glass or metal having the top finished off square and without a spout, and having the internal diameter of 32 ± 2 mm and internal depth of 185 ± 5 mm.
iv. Apparatus for the determination of milk fat: As already described for Gerber method.
2.2 Reagents:
As required for the determination of milk fat by Gerber method.
2.3 Methodology:
A. Preparation of milk sample for lactometer reading. Warm the sample of milk to a temperature of 40 to 45°C and maintain within this range for 5 min during which time the contents of the bottle are adequately mixed. Care shall be taken to avoid the formation of air bubbles or churning of fat when mixing the sample. After this, cool the sample to 27 ± 2°C and held within this range until the specific gravity reading is taken. The sample shall not be held for more than 1 h. The lactometer reading is then taken according to the following procedure.
B. Determination
Invert the sample bottle gently two or three times and then pour down the milk in the lactometer jar along its side so as to avoid the formation of air bubbles. Sufficient milk should be poured into the jar to ensure that some of it overflows when the lactometer is inserted.
The lactometer, held by the stem, is inserted in the sample and released when it is approximately in its position of equilibrium thus avoiding wetting more than a very short length of the stem above the milk surface. As soon as the lactometer is at rest, the scale reading corresponding to the top of the meniscus of milk is noted. The lactometer jar shall be vertical and the bulb of lactometer shall not touch the side. It is advisable to repeat the reading after depressing the lactometer about 3 mm and allowing it to come to rest. Note temperature of milk immediately after taking the lactometer reading with the help of the thermometer. It is generally preferred to take the lactometer reading at 27°C.
Determine the fat content of milk by the Gerber method as described previously. Convert the observed lactometer reading to the corrected lactometer reading (CLR) at 27°C as per the fat percentage and temperature of the milk with the help of Table 2.1. The % of SNF and total solids in milk is then calculated using the following formula:
SNF (in %)=CLR/4+0.25F+0.44
Where
SNF = Solids-not-fat in milk; F = Fat content of milk
CLR = Corrected lactometer reading at 27°C,
Table 2.1: Correction to be applied to lactometer readings taken at temperature other than 27°C to obtain corrected lactometer reading of milk at 27°C.
Temperature Fat Percentage of Milk sample
0 2 4 6 8
19.0 -2.2 -2.4 -2.6 -2.7 -2.9
19.5 -2.1 -2.3 -2.4 -2.6 -2.7
20.0 -2.0 -2.1 -2.2 -2.4 -2.5
20.5 -1.8 -2.0 -2.1 -2.2 -2.3
21.0 -1.7 -1.8 -1.9 -2.0 -2.2
21.5 -1.5 -1.7 -1.7 -1.9 -2.0
22.0 -1.4 -1.5 -1.6 -1.7 -1.8
22.5 -1.3 -1.4 -1.4 -1.5 -1.6
23.0 -1.1 -1.2 -1.3 -1.4 -1.4
23.5 -1.0 -1.1 -1.1 -1.2 -1.3
24.0 -0.8 -0.9 -1.0 -1.0 -1.1
24.5 -0.7 -0.8 -0.8 -0.9 -0.9
25.0 -0.6 -0.6 -0.6 -0.7 -0.7
25.5 -0.4 -0.5 -0.5 -0.5 -0.5
26.0 -0.3 -0.3 -0.3 -0.3 -0.4
26.5 -0.1 -0.2 -0.2 -0.2 -0.2
27.0 0 0 0 0 0
27.5 +0.1 +0.2 +0.2 +0.2 +0.2
28.0 +0.3 +0.3 +0.3 +0.3 +0.4
28.5 +0.4 +0.5 +0.5 +0.5 +0.5
29.0 +0.6 +0.6 +0.6 +0.7 +0.7
29.5 +0.7 +0.8 +0.8 +0.9 +0.9
30.0 +0.8 +0.9 +1.0 +1.0 +1.1
30.5 +1.0 +1.1 +1.1 +1.2 +1.3
31.0 +1.1 +1.2 +1.3 +1.4 +1.4
31.5 +1.3 +1.4 +1.4 +1.5 +1.6
32.0 +1.4 +1.5 +1.6 +1.7 +1.8
32.5 +1.5 +1.7 +1.7 +1.9 +2.0
33.0 +1.7 +1.8 +1.9 +2.0 +2.2
33.5 +1.8 +2.0 +2.1 +2.2 +2.3
34.0 +2.0 +2.1 +2.2 +2.4 +2.5
34.5 +2.1 +2.3 +2.4 +2.6 +2.7
35.0 +2.2 +2.4 +2.6 +2.7 +2.9
Milk is a common drink of our daily diet. But mostly time, it may have Urea, Formalin, Vanaspati, Starch and water as impurity. Packed milk from brands as well as milk purchased from milk-vendors can be adulterated so it is always better to check for them before consuming.
Water turns out to be the most common adulterant in milk. It reduces the nutritional value of milk. If contaminated, water poses a health risk to consumers.
How to check water adulteration in Milk by using lactometer :
A lactometer measures the density of milk. It tells the user how much water is in the milk that is being sampled. This is an instrument used to determine the richness of milk.
Lactometer : Measure the amount of water in the milk. A Lactometer is used to find out the amount of water in the milk. It works on the principle of specific gravity of milk. It consists of a Test-Tube and a Meter Bulb.
Using this instrument is very simple. Put some milk in the test-tube. Dip the meter bulb in it, the bulb going in first. You will notice that the meter bulb floats. The reading on the meter indicates how pure/impure your milk is.
The deeper the bulb sinks, the more dilute/impure the milk. If the reading is at the red mark, it shows that the milk is rich and pure.
Milk is one of the most important ingredients for children’s overall development, it is essential for adult health as well. Milk is also widely used in food item like desserts, baking, tea and coffee etc.
Most of the commonly used adulterant for milk includes Detergent, White Paint, Caustic Soda, Refined Oil and glucose.
The most common reason for milk adulteration is the difference between demand and supply of milk. In order to meet the demand, the suppliers usually adulterate the milk and increase the quantity.
The adulterants like Salt, Detergents and glucose add to the thickness and viscosity of the milk while starch prevents curdling of milk.
These adulterants are hazardous and cause irreversible damage to the human body.
The detergents in milk caused food poisoning and gastrointestinal complications. The other synthetic compounds cause impairments, heart problems and cancer.
Due to lack of hygiene in milk handling and packaging, detergents (used during cleaning operations) are not washed properly and find their way into the milk. Other contaminants like urea, starch, glucose, formalin along with detergent are used as adulterants. These adulterants are used to increase the thickness and viscosity of the milk as well as to preserve it for a longer period.
Water turned out to be the most common adulterant in milk. It reduces the nutritional value of milk. If contaminated (with pesticides, heavy metals), water poses a health risk to consumers
Adulteration of water in milk is so common in India. But it is not much toxic to health as adulteration of drinking water is.
Water Adulteration Test in Milk without Lactometer :
1) Slip Test : To identify adulteration from water, a simple test is milk slip test. Run the drop of milk on smooth or polished surface. Pure milk leaves some residue or traces behind. However, milk mixed with water will simply flow out.
2) Reduction Test : Boil milk on slow heat for 2-3 hours till it solidifies and become hard (khoya). Rock solid, rough residue means the milk is adulterated while oily residue means its of good quality.
3) Checking for Synthetic Milk : Synthetic milk is made by mixing chemicals and things like soap in natural milk. Synthetic milk can be easily identified by bad taste. It feels soapy when rubbed and turns yellowish when heated.
Note :
1) Salt and detergents are added to adjust lactometer reading to add thickness to the milk.
2) Contaminate milk can be countered by ensuring proper sanitation and hygiene at all processing stages as well as by boiling and pasteurizing milk properly.
3) Nitrogenous compounds are added to artificially enhance the taste of protein in milk.
4) Synthetic milk turns yellowish on heating. With some experience, one can easily make out whether it is pure natural milk or a synthetic liquid sold as milk.
5) One of the most common form of adulteration in milk is mixing of urea as it does not changes the taste and is little difficult to detect.
Consideration
1) Make sure you boil the milk on slow flame till boiling point. Avoid re-boiling milk as it brings down the nutritional value of milk.
2) While the easiest way to adulterate milk is by adding water, reagents and prohibited neutralisers like hydrated lime, sodium hydroxide, sodium carbonate or sodium bicarbonate are added to milk to prevent spoilage.
3) One really can not do anything when it comes to the packed pasteurized milk. Such products undergo a series of processes and it is hard to trace the point of adulteration.
4) Since the adulteration techniques have advanced these days so it is very difficult to identify adulteration. You can not find any adulteration until you use some catalyst to check its purity.
5) There were 37 pure Indian breeds and due to foreign activist’s effort almost all the breeds are now extinguished with only few breeds left.
6) Mixing water in milk is a common practice by local milkman. Having harmful elements like Urea, Carbonates and Bicarbonates, salt, sugar, Hydrogen Peroxide, Neutralizers (NaHCO3, Na2 CO3, NaOH, Ca(OH)2 etc.), sodium chloride, etc. added to our everyday drink is completely unacceptable.
To stop milk adulteration near your diary, you should do research and keep an eye. Visit your dairy periodically and keep a strict watch.
Specific gravity of milk:
Specific gravity of milk means its density compared with water. It is the ratio of the weight of equal volumes of milk and distilled water at the same temperature. The specific gravity of milk varies according to the proportion of fat, SNF (solid- not fat) and water. The specific gravity of the whole cow’s milk ranges from (1.028 to 1.034) gm/ml, with an average of (1.032) gm/ml which means that milk is (1.032) times heavier than water. Lactometer is a special hydrometer designed for use with milk graduated in the range of (1.024 to 1.037). The lactometer operates on the principle that a body floating in water is buoyed-up by a force equal to the weight of water displaced at (60) ˚F or (15.5) ˚C, the material in milk have the following specific gravities: water=(1.0)gm/ml, fat=(0.93) gm/ml, solid- not fat= (1.610)gm/ml.
Significance of the test:
1- Partial skimming increases the specific gravity of milk.
2- Adulteration of milk with water lowers the specific gravity.
3- Use the lactometer reading together with the fat content to estimate the SNF and TS (Total solid) content of the milk. It is best to combine the lactometer reading with a fat test.
Procedure of lactometer test:
1-Adjust the milk temperature to near (15.5) ˚C or (60 ˚F).
2- To mix the milk, but to avoid incorporating air.
3- Place enough milk in the jar so that as the lactometer floats.
4- Centre the lactometer in the cylinder and within 30 seconds after mixing read to the top of the milk meniscus. Again take the milk temperature.
5- If the milk temperature is not exactly (15.5) ˚C or (60 ˚F), correct the lactometer reading by adding (0.2) lactometer degree for each degree Celsius above (15.5) ˚C or (0.1)lactometer degree for each degree Fahrenheit above (60) ˚F, also subtract(0.2) lactometer degree for each degree Celsius below (15.5) ˚C or (0.1)lactometer degree for each degree Fahrenheit below(60) ˚F.
Note: must be adding (0.5) degree to lactometer reading to correct surface tension error.
Specific gravity = corrected lactometer reading (L) / 1000 +1+ (1.2 * FAT%)TS% = L / 4
SNF%= L / 4 + (0.2 FAT%) SNF%= TS% – FAT% Milk Moisture % = 100- TS % Example1: Calculate the specific gravity of milk sample if you know the lactometer reading is (31) and milk temperature is(14.5)˚C? Solution: 31 + 0.5 = 31.5 (adding surface tension deference) 15.5 – 14.5 = 1˚C 1 0.2 = 0.2 (subtract from lactometer reading)
31.5 – 0.2 = 31.3(corrected lactometer reading)
Specific gravity = corrected lactometer reading (L) / 1000 +1
= 31.3 / 1000 + 1
= 1.0 313 gm/ml.
Example2:
When lactometer but on milk sample gives reading (32) on (62)˚F, calculate specific gravity , TS% and SNF % , if you know FAT % = (4 %) ?
Solution:
32 + 0.5 = 32.5 (adding surface tension deference)
62 – 60 = 2 ˚F
2 * 0.1= 0.2 (add to lactometer reading)
32.5 + 0.2 = 32.7 (corrected lactometer reading)
Specific gravity = corrected lactometer reading (L) / 1000 +1
= 32.7 / 1000 +1
= 1.0327 gm/ml.
TS % = L / 4 + (1.2 * FAT %)
= 32.7 / 4 + (1.2 * 4)
= 12.975 %
SNF % = L / 4 +(0.2 *FAT %)
= 32.7 / 4 +(0.2 * 4)
= 8.975 %
Or:SNF % = TS % – FAT % = 12.975 – 4 = 8.975 %
Compiled & Shared by- Team, LITD (Livestock Institute of Training & Development)
Image-Courtesy-Google
Reference-On Request.