Lumpy skin disease in livestock and their impact:A Review
DR KRANTI SHARMA
DR MUKESH SHARMA
Abstract
Lumpy Skin Disease (LSD), which affects cattle in Asia, has recently been presented as a dreadful threat. The genus Capripoxvirus, which causes Lumpy Skin Illness (LSD), has been linked to an economically significant transboundary viral disease of livestock that is thought to have originated in Zambia, Africa. LSDV is primarily observed during the rainy season and can be transmitted both by vector and non-vector channels. Cattle and buffalo are the principal species affected, and nodules in the skin and mucous membranes appear along with a high fever. The disease has been spreading in various parts of the country. Despite having a lower mortality rate, this illness has significant economic costs due to decreased milk production, abortions, infertility, and other complications. Although the disease is still relatively new to India, it has already spread across a sizable portion of the country. Only supportive care is used to treat the disease once it has been contracted, however immunization, herd biosecurity, vector management, and isolation of the afflicted animals are said to be effective methods of control and prevention. Future research should focus on understanding the true impact of LSD on livestock and its potential risk factors from a geographic perspective.
Introduction
An infectious condition known as lumpy skin disease (LSD) in cattle is brought on by the Neethling virus, a member of the Poxviridae family of viruses. The disease is called by various names such as LSD, seudo-urticaria, Neethling virus disease, exanthema nodularis bovis, etc.
(Al-Salihi, 2014; OIE, 2017). Fever, swollen superficial lymph nodes, and many nodules on the skin and mucous membranes (including those of the respiratory and digestive tracts) with a diameter of 2 to 5 cm (1-2 in) are the disease’s characteristics (Sevik et al., 2016). It is classified as a trans boundary disease with only a few hosts mainly ruminants e.g. water buffalo and cattle, sheep, and goats even in close contact with infected cattle were found unaffected in Africa (Davies, 1991). The younger animals are found to be more susceptible to the virus (Tageldin et al., 2014). The non-ruminants are not affected by the virus (Shen et al., 2011). Infected cattle may also develop lameness and edematous swelling in their limbs. The virus has significant economic ramifications because it frequently causes permanent skin damage in animals, decreasing the market value of their hide. Additionally, the condition frequently results in long- term impairment, monetary loss, decreased milk production, stunted growth, infertility, abortion, and sometimes even death. The lumpy skin disease virus is the infectious cause of lumpy skin
disease (LSD) (LSDV) (OIE, 2021; Tuppurainen et al., 2017). Being a vector-borne disease too, insects like mosquitoes, flies (Culicoides), and ticks are mainly responsible for transmission but other sources like contact, water, and feed can also cause the virus transmission (EFSA Panel, 2015). Numerous consequences, including mastitis and metritis, emerge as a result of the disease’s high fever and immunological weakness, seriously affecting cow reproductive and output. (Tuppurainen and Oura, 2012). LSD was first noted in Zambia, Africa, and was afterwards noted in North Sudan and Southern Africa. Israel was the first country outside of Africa to do so in 1989, and then Bahrain, Kuwait, Oman, Yemen, Lebanon, and Jordan in the Middle East (FAO, 2013). In the context of the subcontinent, LSD was first discovered in India in August 2019. From there, an open border and livestock smuggling nexus spread the disease to Nepal, where it was discovered in the Morang area of Eastern Nepal in June 2020 (Gupta et al., 2020; Oie-Wahis, 2021). This review aims to provide information regarding the disease scenario in India based on the OIE database, potential risk factors, and effects on the economy and livestock.
Viral biology of lumpy skin disease
The largest viruses that can naturally cause disease in most domestic animals, with the exception of dogs, are found in the family Poxviridae. Entomopoxvirinae, which includes poxviruses that infect insects, and Chordopoxvirinae, which includes poxviruses that infect vertebrates, are its two subfamilies (Quinn et al., 2016). The Poxviridae family is distinguished by its extensive and intricate genome, which consists of a single, linear molecule of double-stranded DNA (ds DNA) that codes for about 200 proteins. The ends are joined together to form a continuous DNA molecule with no loose ends. The only DNA viruses that are known to finish their replicating cycle in the cytoplasm are pockviruses. Viral enzymes play a major role in the mediation of both processes. In the cytoplasm, the dsDNA serves as a template for the creation of mRNA (for the translation of proteins) and copies of the genome for progeny virions. The mechanism underlying virion assembly is largely unknown since virions are huge and complex. By budding, viruses are expelled from the cell. Cross-reactivity between species is explained by the presence of at least 10 main antigens in the Poxviridae families, each of which has a shared nucleoprotein antigen. The virus particle contains at least 10 viral enzymes, many of which are involved in nucleic acid metabolism and genome replication (Carter et al., 2005).
The virus is arranged with a 151-kbp genome consisting central coding region surrounded by inverted terminals and has 156 putative genes, however, in terminal regions, the collinearity of the genome is disrupted with low or absent amino acid identity (Tulman et al., 2001). The virus is stable and can withstand ambient temperature for a comparatively long time, survive in organic matter, and can be detected from the nodules of skin even after 30 days or more. Although the titer of the virus doesn’t change with exposure to the pH range from 6.6-8.6 for 5 days at 37oC it is susceptible to even moderate chemical changes with both alkaline or acidic pH and may reduce the virus load and the chemicals like ether, chloroform, formalin, phenol, per oxygen compounds, the essential oil may decrease the virus load (OIE, 2017).
Transmission of LSDV
Lumpy skin disease viruses are reported to be transmitted by various routes like direct and indirect contact, arthropod transmission, and seminal transmission (Tuppurainen, 2015). Various arthropod is known to be a vector for transmission of the LSDV and the presence of the virus in those vectors was seen (Chihota et al., 2003). Sexual transmission was observed with LSD virus through infected semen of bull to the heifer and even congenitally to the fetus (Annandale et al., 2014; Irons et al., 2005).
The infected bull may transfer the virus to adult females as well through natural insemination or artificial insemination and the infected female may give birth to calves with skin lesions (Tuppurainen et al., 2017). Sharing common grazing areas, feeding, watering method, and reduced movement of the animal were reported as risk factors for LSD transmission (Gari et al., 2010). According to Das et al. (2021), there are numerous potential routes via which LSDV might spread to cattle, demonstrating the critical role biosecurity can play in avoiding it.
Various methods of transmission
Another attempt to spread LSDV by handling infected animals manually before coming into contact with vulnerable calves or by housing uninfected and infected animals in the same area was unsuccessful. This suggests that contact between infected and susceptible animals, whether direct or indirect, is a poor mechanism of transmission (Tuppuraine et al., 2017). LSDV has been isolated from the semen of experimentally infected bulls, although transmission of LSDV through semen (natural mating or artificial insemination) has not been experimentally established in prior publications (Irons et al., 2005). On the other hand, a recent study by Annandale et al. (2014) shown that experimental transmission of LSDV via semen from infected cattle is feasible; however, additional research is required to determine whether this also happens during natural mating or artificial insemination.
Vectors function
LSDV can be mechanically transmitted by a number of hematophagous arthropod vectors, according to evidence from several sources. Similar to how high morbidities are observed in warm, humid climates with high mosquito populations, with 50–60% attack rates, and low morbidities of 5–15% in arid areas with low mechanical vector densities (Tuppurainen et al., 2012; Au- Ibar et al., 2013; Ali H et al., 2012). Recent investigations on ticks have revealed mechanical or intrastadial transmission by Rhipicephalus appendiculatus and Amblyomma hebraeum, as well as transstadial and transovarial persistence of LSDV in Rhipicephalus decoloratus, Rhipicephalus appendiculatus, and Amblyomma hebraeum (Tuppuraine et al., 2011; Lubinga et al., 2014). On the other hand, female Aedes aegypti mosquitoes have been used in
experiments to show mechanical transmission of the LSDV; however, clinical sickness was generally minor in most of the animals exposed to infected mosquitoes (Chihota et al., 2001).
Without actually replicating in the arthropod cells or tissues, the virus is spread mechanically through the mouth portions of vectors that have been tainted. Aedes aegypti has been implicated in long-distance airborne transmission in disease-free areas, which is thought to challenge the mobility restriction management strategies (Tuppuraine et al., 2017). The virus has also been found in the Stomoxys, Biomyia, Musca, Culicoides, and Glossina species, all of which voraciously feed on domestic cattle and may be capable of spreading LSD (Carn and Kitching, 1995). Despite the fact that the virus has been found in Anopheles stephensi, Culex quinquefascuatus, Stomoxy calcitrans, and Culicoides nebeculosis, attempts to mechanically transmit LSD to susceptible animals have been unsuccessful (Chihota et al., 2003). Sevik and Dogan recently looked at the possible involvement of Culicoides species in the spread of LSDV, and they found that Culicoides punctatus may have contributed to the outbreak that occurred in Turkey in 2014–2015 (Sevik and Dogan, 2015). It follows that different arthropods that graze on cattle are capable of spreading the LSDV virus.
Pathogenesis
There have been few studies conducted on the pathogenesis of LSD in cattle (El-Kenawy and El- Tholoth, 2010). Fever and viremia are the first symptoms of the generalized type, which is then localized to the skin and produces inflammatory nodules (Constable et al., 2016). Following Subcutaneous or intradermal inoculation of cattle with LSDV, localized swelling at the site of inoculation developed 4 to 7 DPI which is varying in size from 1 to 3 cm and covering up to approximately 25% of the skin surface. 7 to 19 DPI are typically followed by enlargement of the local lymph nodes and widespread development of cutaneous nodules. Viremia and Low levels of viral shedding in oral and nasal secretions was detectable between 6 and 15, and 12 and 18 DPI, respectively following febrile reaction. LSDV is also demonstrated in saliva, semen and skin nodules for at least 11, 42 and 39 days after the development of fever, respectively (Coetzer and Tuppurainen, 2004). Vasculitis and lymphagitis are caused by viral replication in microphages, fibroblasts, pericytes, endothelial cells, and likely other cells in blood vessel and lymph vessel walls in afflicted locations, while thrombosis and infarction may occur in severe cases (Coetzer and Tuppurainen, 2004). Very young calves, breastfeeding cows, and underweight animals appear to suffer more severe disease in natural infection, which may be caused by a reduced humoral immunity. Antibodies was detectable 21 DPI using serum
neutralization tests (Babiuk et al., 2008). After recovering from a natural infection, immunity lasts a lifetime; calves of immune cows pick up maternal antibodies and exhibit clinical disease resistance for roughly six months (Al-Salihi, 2014). Affected animals eventually recover from the virus, and LSDV has no known carrier condition (Tuppuraine et al., 2017).
Hazard factors
The morbidity rate ranges from 10 and 20% whereas mortality rates are generally 1 to 5%. Typically, the incubation stage lasts one to four weeks (Tuppurainen and Oura, 2012). Das et al. (2021) in their review have classified the risk factor of disease into three categories as host- associated factors, agent related factors and environment, and management factors and have stated that LSDV can be isolated from various exudations of body, saliva, blood, milk, and semen. For conditions like that of India, similar risk factors can play role in the spread of the virus. Open borders with India and warm climatic conditions can be important risk factors for India. With the increase in demand for milk products in India, the population of high yielding cross breed cattle is also increasing in trend and it has increased the risk of spread of the diseases, many studies have suggested that cross-breed animals are more susceptible to the infection (Paudel et al., 2021; Das et al., 2021). The diseases are susceptible to all breeds and sex of cattle and buffalo but Bos taurus which generally has thin skin and is high milk-producing animals are more vulnerable (Gumbe, 2018)
Clinical signs and symptoms
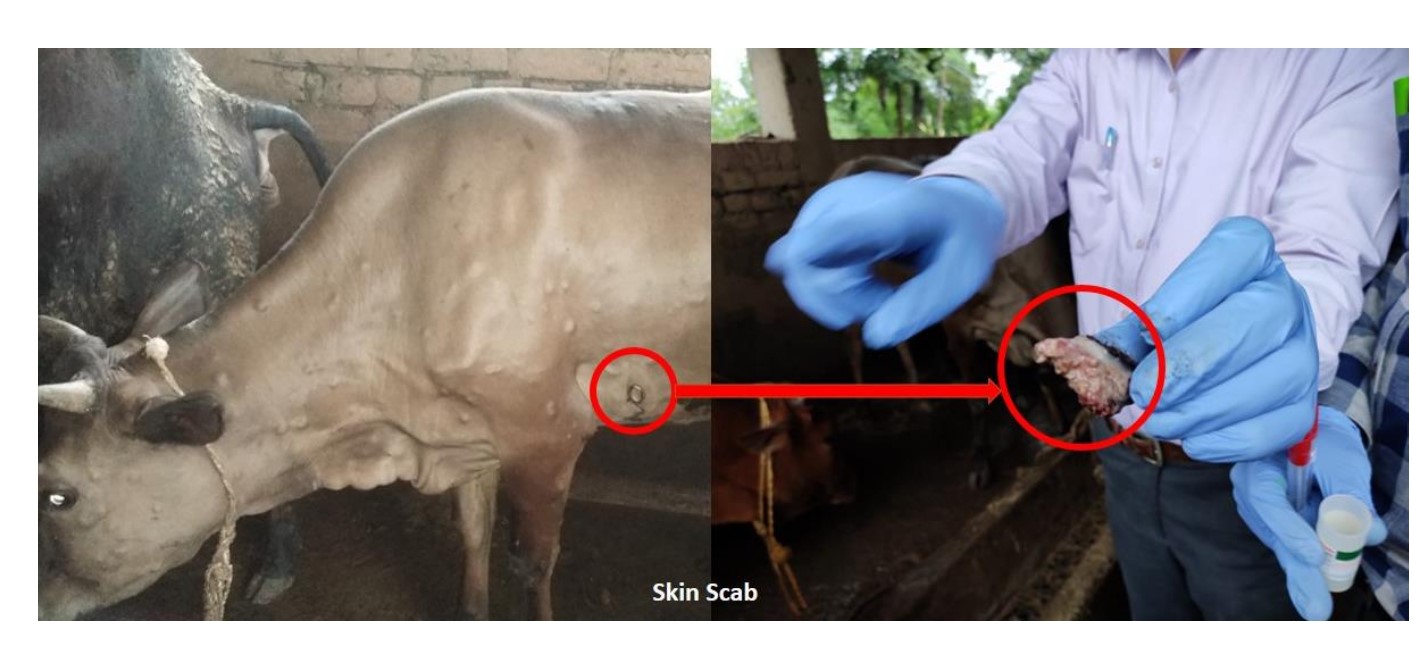
In experimentally infected cattle, regardless of the route of infection, the interval between inoculation and the initial observation of widespread clinical indications spans from 7 to 14 days (Carn and Kitching, 1995), but in natural cases, it is between 2 and 5 weeks (Tuppuraine et al., 2005). LSD can be classified into mild and severe forms based on the number of lumps (nodules) and occurrence of complications, dose of the inoculum as well as the susceptibility of the host and the density of insect population. Accordingly appearance of one or two lumps or nodules within 2 days after onset of the fever (1 to 5 cm in diameter), depression, anorexia, excessive Clinical signs of slightly impacted cattle include salivation, ocular and nasal discharge, agalactia, and emaciation. Nodular lesions, which are painful and hyperemic, may also appear on the animal’s body skin. These lesions are most common on the animal’s snout, nares, back, legs, scrotum, perineum, eyelids, lower ear, nasal mucosa, and oral mucosa (Salib snd Osman, 2011). Serious depression, anorexia, and a characteristic number of (more than hundreds) nodules, which are typically quite consistent in size in the same animal, are seen throughout the animal’s body in severe cases that may last for 7–12 days.
Diagnosis
The lumpy skin disease can be identified based on the clinical signs and symptoms shown by the animals. The severity, duration, and nature of the symptoms will depend on the health of the animal. Several state-of-the-art methods can be used in a laboratory setting to make a confirmatory diagnosis, including virus isolation and culture, serological testing, polymerase chain reaction (PCR), dot blot hybridization (DBH), agar-gel immune diffusion test (AGID), indirect enzyme-linked immunosorbent assay (ELISA), direct fluorescent antibody test, west blotting, routine histopathology, and immune histopathological staining (Awad et al., 2010; OIE, 2021; Tuppurainen et al., 2005). In the differential diagnosis of the disease ringworm, pseudo- cowpox, vaccinia virus, stomatitis, bovine herpes mammalities, dermatophilosis, insect bites, and cutaneous tuberculosis should all be taken into consideration. (Das and colleagues, 2021; Gumbe, 2018).
Treatment for the virus that causes lumpy skin
Lumpy Skin Disease is only symptomatically treated, and antibiotic medication is used to stop ubsequent bacterial problems (Abutarbush et al., 2013). A mixture of antimicrobials, anti- inflammatory drugs, supportive care, and anti-septic treatments have been successfully used in treatment studies by Salib and Osman with the goal of reducing LSD consequences and saving lives (Salib and Osman, 2011). The trial’s problems, such as ocular opacity (keratitis), mastitis, diarrhoea, lameness, pneumonia, and myasis, all went away in 3 to 2 weeks (Gari et al., 2010). However, treating LSD (and its complications) is expensive and does not always result in full recovery; for these reasons, prevention is preferable to mitigating the significant economic losses brought on by hide damage, milk loss from mastitis, and loss of animal product due to death, abortion, fever, and myiasis. The significance of vaccination in managing lumpy skin diseases (LSD) in endemic areas is shown on the epidemiological aspects and economical impact of lumpy skin diseases in Ethiopia (Gari et al., 2010). The authors also note that immunisation can lower LSD-related costs by 31 percent per head for Holstein Friesian or crossbred herds and by
17 percent per head for local zebu herds. As a result, movement limitations and the killing of infected animals alone are typically ineffective in controlling the disease in endemic areas. Effective LSD vaccinations are available, and the sooner they are employed, the less severe an outbreak’s expected economic effects will be (Tuppuraine et al., 2017). The capripoxvirus family is recognised to offer cross protection. Therefore, both homologous (using the Neethling LSDV strain) and heterologous (using the sheep pox or goat pox virus) live attenuated immunizations can protect cattle against LSD infection (OIE, 2013). Among the capripoxvirus (CaPV) vaccine strains that are offered commercially are the LSDV Neethling strain, the Kenyan sheep and goat pox virus (KSGPV) O-240 and O-180 strains, the Yugoslavian RM65 sheep pox (SPP) strain, the Romanian SPP strain, and the Gorgan goat pox (GTP) strain (Abutarbush et al., 2017).
Conclusion
Small-scale farmers are currently in grave danger due to the disease. Up until the eighteenth century, greater Africa was the disease’s primary distribution area. From there, it migrated to the Middle East, Eastern Europe, the Russian Federation, and, more recently, Asia. The recurring LSDV attacks in open areas have been noted by the scientific community. Therefore, it should go without saying that now is the ideal time to prepare for emergencies in order to stop the considerable spread of this trans-boundary disease. Focus should be put on vector control, movement limitations, severe quarantines, improved vaccination programs, suitable veterinary treatment, and overall farm sanitary management in order to stop the incursion and transmission of the disease. The work thus encourages additional investigation into the ecology and epidemiology of LSDV in India, as well as the molecular characterization and identification of the causal agent.