LUMPY SKIN DISEASE (LSD): AN EMERGING TRANSBOUNDARY VIRAL DISEASE
Jatav*, Y. Verma, A. Dubey, M. Swamy and P. Verma
Department of Veterinary Pathology
College of Veterinary Science and AH, Jabalpur,
Nanaji Deshmukh Veterinary Science University
Jabalpur (M.P.), India
Introduction: Lumpy skin disease is a vector borne pox disease of cattle and Asian water buffaloes characterized by the appearance of skin nodule (FAO, 2017). It is an infectious, re-emerging, transboundary disease of bovine caused by Lumpy skin disease virus (LSDV). In current scenario this disease has emerge as a devasting threat for the large ruminants (Das et al., 2021). The disease cause socio-economic losses due to re-emerging transmission to small scale and courtyard agrarians (Negasso et al., 2016). Considering the disease burden, morbidity and mortality, cattle are found as more sensitive to the infection compared to buffaloes and other ruminants (Kardjadj et al., 2016). This disease is devasting because it causes a dramatic decline in milk yield, poor skin condition and sterility in bulls (Angelova et al., 2018). In addition to vectors, transmission may occur through consumption of contaminated feed or water, direct contact, natural mating or artificial insemination. Despite this economic importance of this disease, trace amount of literature available on this disastrous vector borne disease in various parts of India. Recurrent outbreaks and reappearance of this disease in various states of India identified the importance of reassess of the disease biology, spread, pathogenesis and bring up to date preventive and control policy. Taking into account, a systemic review of Lumpy Virus Disease has been conducted.
Epidemiology:
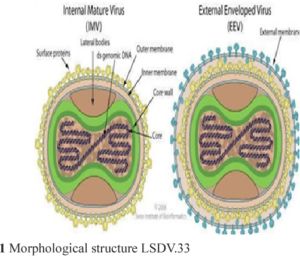
Causative agent: Lumpy skin disease is caused by the lumpy skin disease virus (LSDV) belongs to genus Capripoxvirus (CaPV) and family Poxviridae. This virus shares the genus with sheep pox virus and goat pox virus, which are closely related by phylogenetically distinct (FA0, 2017). This virus is an enveloped, linear, ovoid, double stranded DNA virus which is very stable and little genetic variable. Characteristic of this revealed, impermeable to physical and chemical agents and remains stable between pH 6.6 to 8.6, but is susceptible to higher alkaline conditions. This virus remains survive for 33 days in necrotic skin nodules, 35 days in desiccated crusts, up to 6 months in sunlight infected tissue and minimum 18 days in air dried hides at room temperature (Das et al., 2021).
Fig: Classification of LSDV and morphologic structure of LSDV
Geographic distribution: According to the OIE, India faced three primary outbreaks of LSD at Mayurbhanj district in the state of Odisha, followed by one incursion each at four more districts, bringing the total number of outbreaks in the Eastern share of the country. There were 182 clinically affected among 2539 susceptible animals accounted for the apparent morbidity rate 7.1% with no recorded mortalities. In terms of districts affected, Cuttack displayed the highest morbidity rate of 38.34%, and Kendrapara showed 0.75% (Sudhakar et al., 2018).
Fig: Distribution of LSDV in India
- Susceptible Hosts: LSDV is highly host specific and causes diseases only in cattle (Bos indicus and B. taurus) and water buffalo (Bubalus bubalis). There is evidence from a study in Ethiopia of differential breed susceptibility to LSD, with Holstein Friesian or crossbred cattle exhibiting higher morbidity and mortality due to LSD when compared with local zebu cattle (OIE, 2017)
- Transmission: The principal means of transmission is believed to be by arthropod vector. Though no specific vector has been identified to date, mosquitoes (e.g. Culex mirificens and Aedes natrionus), biting flies (e.g. Stomoxys calcitrans and Biomyia fasciata) and male ticks (Riphicephalus appendiculatus and Amblyomma hebraeum) could play a role in the transmission of the virus. The importance of different 2 arthropod vectors is likely to vary in different areas depending on the abundance and feeding behaviour of the vector. Infected bulls can excrete the virus in the semen, however transmission of LSD via infected semen has not been demonstrated. It is not known if transmission can occur via fomites, for example ingestion of feed and water contaminated with infected saliva. Animals can be infected experimentally by inoculation with material from cutaneous nodules or blood. Direct contact is considered to play a minor, if any, role in the transmission of the virus (OIE, 2017).
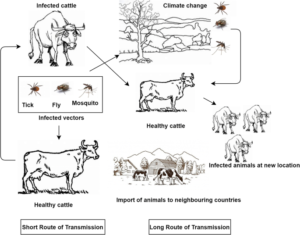
Fig: Transmission of LSDV
- Sources of infection: Skin nodules, scabs and crusts contain relatively high amounts of LSDV. Virus can be isolated from this material for up to 35 days and likely for longer. LSDV can be isolated from blood, saliva, ocular and nasal discharge, and semen. LSDV is found in the blood (viraemia) intermittently from approximately 7 to 21 days post-infection at lower levels than present in skin nodules. Shedding in semen may be prolonged; LSDV has been isolated from the semen of an experimentally infected bull 42 days post-inoculation. There has been one reported of placental transmission of LSD. LSD does not cause chronic disease. It does not exhibit latency and recrudescence of disease does not occur.
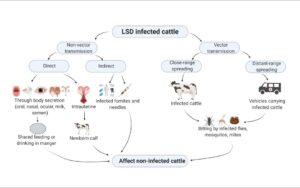
Fig: Sources of LSDV infection
Pathogenesis: Following LSDV infection, virus replication, viremia, fever, cutaneous localization of the virus and development of nodules occur (Constable et al., 2017) Viremia occurs after the early febrile condition for almost 4 days. Following skin lesions due to the replication of the virus in certain cells such as fibroblasts, pericytes, and, endothelial cells of lymphatic and blood vessels lesions are produced in those sites (Das et al., 2021).
Diagnosis:
Clinical Signs:
Depression, anorexia and emaciation, High rise of body temperature that may exceed 41°C, marked reduction in milk yield in lactating cattle, Rhinitis, conjunctivitis and excessive salivation, Enlarged superficial lymph nodes, cutaneous nodules of 2–5 cm in diameter develop, particularly on the head, neck, limbs, udder, genitalia and perineum within 48 hours of onset of the febrile reaction. These nodules are circumscribed, firm, round and raised, and involve the skin, subcutaneous tissue and sometimes even the underlying muscles. Large nodules may become necrotic and eventually fibrotic and persist for several months. Small nodules may resolve spontaneously without consequences. Myiasis of the nodules may occur, Vesicles, erosions and ulcers may develop in the mucous membranes of the mouth and alimentary tract and in the trachea and lungs. Limbs and other ventral parts of the body, such as the dewlap, brisket, scrotum and vulva, may be edematous, causing the animal to be reluctant to move. Bulls may become permanently or temporarily infertile. Pregnant cows may abort and be in anestrus for several months. Recovery from severe infection is slow due to emaciation, secondary pneumonia, mastitis, and necrotic skin plugs, which are subject to fly strike and shed leaving deep holes in the hide (Mulatu and Feyisa, 2018).
Fig: Nodular formation on skin of cattle
Post-Mortem lesions: Skin nodules are usually uniform in size, firm round and raised and may coalesce in to large irregular, circumscribed plaques contain reddish gray and edematous layer. Circular necrotic lesions observed in different visceral organs. Enlarged, edematous lymph nodes and having pyemic foci in addition to cellulitis. Pleuritis and enlargement of medialstinal lymph nodes in severe cases observed. The LSD typical nodular lesions also encompass the musculature and the fascia over limb and appear gray-white surrounded by inflammatory tissue and formation of ulcer. Secondary bacterial infection in severely infected animals showed bacterial pneumonia, tracheal stenosis and chronic orchitis, mastitis, similar inflammatory lesions in female reproductive system (Mulatu and Feyisa, 2018).
Laboratory Diagnosis:
Isolation and identification of organism:
Identification of the agent · Polymerase chain reaction (PCR) is the least expensive and quickest method for detection of LSDV. Skin nodules and scabs, saliva, nasal secretions, and blood are suitable samples for PCR detection of LSDV. · Virus isolation (VI) followed by PCR to confirm the virus identity takes longer and is more expensive but has the advantage of demonstrating the presence of live virus in the sample. · Electron microscopy can be used to identify the classic poxvirus virion but cannot differentiate to genus or species level.
Serological tests: It is not possible to distinguish the three viruses in the Capripoxvirus genus (Sheeppox virus, Goatpox virus and LSD) using serological techniques.
Virus neutralisation: this is currently the gold standard test for the detection of antibodies raised against capripoxviruses.
Western blot: highly sensitive and specific but expensive and difficult to perform.
Capripoxvirus antibody enzyme-linked immunosorbent assay: new commercial kits for detection of capripoxvirus antibodies are currently being developed and released on to the market.
Differential Diagnosis:
Differential diagnosis Severe LSD is highly characteristic, but milder forms can be confused with the following: Bovine herpes mammillitis (bovine herpesvirus 2) (sometimes known as pseudo-lumpy skin disease) , Bovine papular stomatitis (Parapoxvirus), Pseudocowpox (Parapoxvirus) Vaccinia virus and Cowpox virus (Orthopoxviruses), Dermatophilosis, Demodicosis , Insect or tick bites, Besnoitiosis, Rinderpest, Hypoderma bovis infection Photosensitisation, Urticaria, Cutaneous tuberculosis.
Economic importance: This disease is not associated with high mortality, but causes higher economic losses. It results in great economic losses due to reduced feed intake, milk production, weight conversion and hampers the international trade.
Treatment, Prevention and Control: The treatment of LSD is only symptomatic and only targeted at preventing secondary bacterial infections using antimicrobial therapy. The treatment of LSD is costly as well as does not ensure full recovery, therefore prevention is more beneficial to avoid the substantial economic losses due to hide damages, loss of milk due to mastitis and loss of animal product due to death, abortion, fever and myiasis. Prophylactic immunization with homologous (Neethling strain) or heterologous live attenuated vaccine (Sheep/Goat pox vaccine) is the best medical prophylaxis for LSD. Recently India developed Lumpy-Pro Vac Ind to prevent lumpy skin disease in animals. In addition to vaccination other sanitary prophylactic measures are helpful in the control of LSD in domestic animals. These include movement control, restricted grazing, stamping out of severely affected animals, disposal of infected carcass, washing of contaminated premises with disinfectant, use of repellents, strict quarantine and finally, disease awareness programs targeting farmers, veterinarian, animal handlers and persons who direct or indirectly conjoint with animal husbandry activities (Das et al., 2021)
Fig: LSDV vaccine prophylaxis
References:
- Angelova T, Yordanova D, Krastanov J, Miteva D, Kalaydhziev G, Karabashev V, et al. Quantitative and qualitative changes in milk yield and cheese-making properties of milk in cows vaccinated against lumpy skin disease. Macedonian Journal of Animal Science. 2018;8(2):89-95.
- Das, Moumita, et al. “An updated review on lumpy skin disease: Perspective of southeast asian countries.” Adv. Biotechnol. Exp. Ther4.3 (2021): 322-333.Kardjadj M. Capripoxviruses: Transboundary Animal Diseases of Domestic Ruminants. Annals of Virology and Research. 2016;2(3):1024.
- Mulatu E, Feyisa A. Review: Lumpy Skin Disease. Journal of Veterinary Science & Technology. 2018; 09:1-8.
- Negesso G, Hadush T, Tilahun A, Teshale A. Trans-Boundary Animal Disease and Their Impacts on International Trade: A Review. Academic Journal of Animal Diseases. 2016; 5:53–60.
- Sudhakar SB, Mishra N, Kalaiyarasu S, Jhade SK, Hemadri D, Sood R, et al. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transboundary and Emerging Diseases. 2020; 67:2408-2422.
- Tuppurainen, E., T. Alexandrov, and D. J. F. A. P. Beltrán-Alcrudo. “Lumpy skin disease-a manual for veterinarians.” FAO Animal Production and Health Manual20 (2017).
- World Organization for Animal Health (2017) – Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE, Paris.