NEW CASTLE DISEASE: PRESENT SCENARIO
Dr. Jupi Talukdar1, Dr. Monoshree Sarma2, Dr. Mrinmoyee Sarma3and Dr. M. Bindu Pushpa4
- Assistant Professor, Department of Veterinary and Animal Husbandry Extension Education, Institute of Veterinary Science and Animal Husbandry, Siksha ‘O’ Anusandhan, Deemed to be University, Bhubaneswar, Odisha-751030.
- Assistant Professor, Department of Veterinary Pharmacology and Toxicology, Institute of Veterinary Science and Animal Husbandry, Siksha ‘O’ Anusandhan, Deemed to be University, Bhubaneswar, Odisha-751030.
- Assistant Professor, Department of Veterinary Public Health and Epidemiology, Institute of Veterinary Science and Animal Husbandry, Siksha ‘O’ Anusandhan, Deemed to be University, Bhubaneswar, Odisha-751030.
- Assistant Professor, Department of Veterinary Public Health and Epidemiology, Institute of Veterinary Science and Animal Husbandry, Siksha ‘O’ Anusandhan, Deemed to be University, Bhubaneswar, Odisha-751030.
Introduction
New castle disease (ND), a highly pathogenic disease of birds including domestic poultry is worldwide in distribution. It is a devastating disease of poultry having severe economic and social impact on the community rearing it. Historically, the ND is also known as Ranikhet disease by virtue of its place of origin in India.
Occurrence
In the early 20th century, with the introduction of large-scale commercial poultry farming ND was identified. ND is considered as a worldwide menace and its occurrence is endemic in many third world countries of the globe. The disease manifests itself in three forms: lentogenic (low virulence), mesogenic (moderate virulence) and velogenic (highly virulent). On the basis of its predilection sites velogenic form can either be viscerotropic or neurotropic (Alexander, 2000). Velogenic ND may cause 100% mortality in poultry bringing about profound impact on trade restrictions and embargoes in areas reporting outbreak of the said the disease. ND in its virulent form is listed in the World Organization for Animal Health (WOAH) Terrestrial Animal Health Code and should be reported to the WAOH (WAOH Terrestrial Animal Health Code).
The economical impact of ND is enormous owing to large scale mortality and morbidity associated with it. It causes a huge lose to rural community in developing countries who are traditionally involved in backyard chickens farming for income and for protein source. The disease is endemic in under developed nations where agriculture is considered as the core source of national income. Newcastle disease virus (NDV) belongs to paramyxovirus under the family Paramyxoviridae. In recent years, NDV has lured the virologists not only because of its pathogenic potential, but also for its oncolytic activity and its use as a vaccine vector for both humans and animals. The NDV based recombinant vaccine offers a pertinent choice for the construction of live attenuated vaccine due to its modular nature of transcriptionminimum recombination frequency, and lack of DNA phase during replication. Etiology of the disease is a virus under the family paramyxoviruses.
NDV is a member of the genus Avulavirus and belongs to the subfamily Paramyxovirinae under the family Paramyxoviridae. NDV is a pleomorphic enveloped virus that is around 200–300 nm in diameter. The genome of NDV is a negative sense, non-segmented, single stranded RNA virus. The NDV strains that has been isolated from different parts of the world are classified into three genome size groups: 15,186 nucleotides (nt) long are found in the isolates before 1960; 15,192 nucleotides (nt) long in the isolated from China, and 15,198 nucleotides (nt) long discovered in the avirulent strain from Germany (Czegledi et al., 2006, de Leeuw and Peeters, 1999, Huang et al., 2004a, Krishnamurthy and Samal, 1998, Romer-Oberdorfer et al., 1999).
Clinical signs
Clinical manifestations vary significantly based on the APMV-1 strain and bird host factors (species, age, immunity, etc.). However, there are no pathognomonic clinical signs for Newcastle disease. It has rapid onset and signs become apparent throughout the flock in as early as 2 days (average 4–6) following aerosol exposure. Transmission is slower in cases where the primary means of transmission is faecal-oral, and particularly in the case of caged birds. Young birds are more prone to acquire the infection. The clinical signs depend on whether the infecting virus has a predilection for respiratory and digestive systems (viscerotropic) or nervous systems (neurotropic).
Clinical signs of infection with viscerotropic velogenic NDV in chickens include:
- Lethargy
- Inappetence (anorexia)
- Respiratory distress (sometimes birds make a whistling sound)
- Clear mucus discharge from the mouth
- Prostration
- Sudden death (sometimes the only finding)
- Up to 100% morbidity and mortality, especially in naive populations
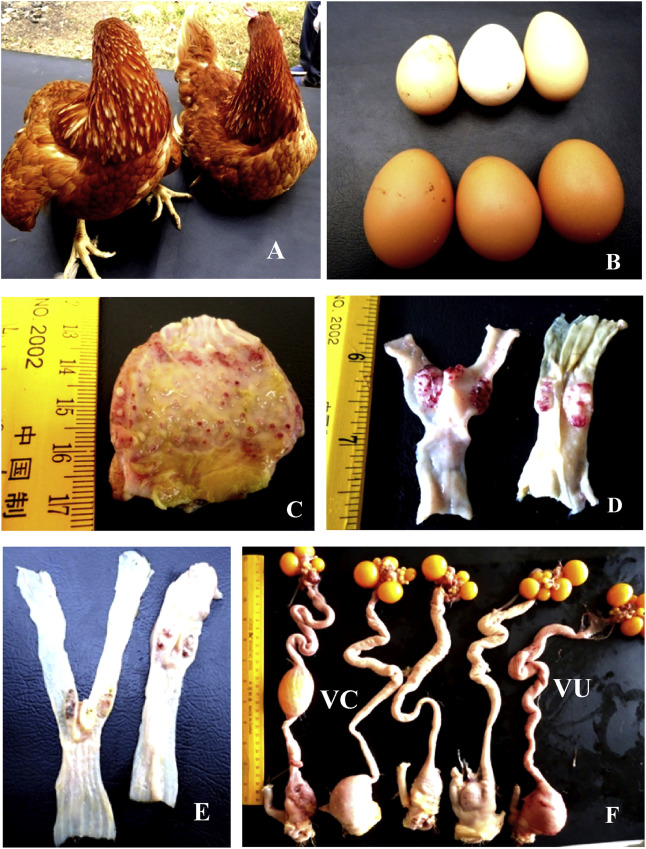
Periorbital and head edema develop in a few cases. The comb might become blue due to hypoxia, and some strains may cause hemorrhage. Birds that survive several days after infection may expel watery greenish diarrhea and show nervous signs including tremor, twisted necks (torticollis), and opisthotonus (especially in vaccinated poultry). Egg production may decrease or completely stop. Eggs may have abnormal color, shape, or surface and albumen becomes watery (may be the only sign in fully vaccinated hens). Well-vaccinated birds may appear clinically normal apart from decreased egg production; however, these birds will continue to shed virus in saliva and feces. Poorly and sub optimally vaccinated birds may develop neurologic signs 10–14 days after infection and may recover.
Clinical findings of infection with neurotropic velogenic NDV in chickens include:
- Respiratory distress with sneezing, coughing, and nasal discharge.
- Tremors and convulsions
- Paralysis of wings and legs
- Torticollis
- Circling with colonic spasms
- Complete paralysis
- Up to 100% morbidity and 50% mortality (up to 90% in young birds)
Clinical signs of infection of chickens with mesogenic NVD include:
- Respiratory signs with gasping, coughing, sneezing, and rales in young birds
- Decrease in egg production, which returns to normal in a few weeks
- Neurologic signs in protracted cases
- Low mortality (may be higher in young birds)
Infections with low virulence NDV vary from in apparent to an onset with mild respiratory signs, but secondary infections may exacerbate the clinical presentation. Adult birds generally suffer from subclinical infection but young birds may show gasping, coughing, sneezing, and rales. Clinical signs in turkey are similar to that of chickens but often are milder. Decrease in egg production is typical sing of the disease, and eggs have soft shells and abnormal shape. Conjunctivitis, rhinitis, difficult breathing (dyspnea), diarrhea, and nervous signs (tremors, ataxia, and torticollis) are typical in pigeons. Mortality can be upto 40%. Infection in cormorants and exotic birds (particularly psittacines) is characterized by difficulty in flying and leg and wing paralysis. Infection of chickens with some pigeon- or cormorant-adapted NDV strains may cause neurologic clinical signs but is not visible in most cases.
Lesions
Only in infection with viscerotropic velogenic Newcastle disease viruses, remarkable gross lesions are usually present. Petechiae may be seen on the serous membranes; hemorrhages of the proventricular mucosa and intestinal serosa are accompanied by multifocal necrotic hemorrhagic areas on the mucosal surface of the intestine, particularly at lymphoid foci such as caecal tonsils and Peyer’s patches. Splenic and thymic necrosis, hemorrhages, and edema may also be present.
In chickens, congestion and hemorrhage of the trachea and lung are seen with velogenic viscerotropic Newcastle disease virus. Egg yolk peritonitis with atrophied follicles may be seen in laying birds. In contrast, birds infected with low virulence NDV strains develop congestion and mucoid exudates in the respiratory tract with opacity and thickening of the air sacs. Secondary bacterial infections increase the severity of respiratory lesions. Hyperemia and mild multifocal petechial hemorrhages are seen in the meninges of the encephalon in infection of chickens with cormorant adapted NDV strains.
Transmission
ND is transmitted through ingestion or inhalation of manure or respiratory secretions from infected birds. The virus gets transferred via fomite materials such as contaminated feed, water, shoes, clothing, vehicles, poultry crates and egg trays, and, especially, moist fecal material. Transmission also takes place from infected eggs to hatching chicks for some APMV (Avian Paramyxovirus ) strains. Incubation period of ND is 2-15 days, with an average of 2-6 days in chickens exposed to velogenic strains. Gallinaceous birds typically keep shedding virus for 1-2 weeks. However, psittacine species such as parrots, macaws, and parakeets shed APMV-1 (Avian Paramyxovirus-1) for months to more than a year.
Prevention
Biosecurity
It is the most important protection strategy. Practices such as avoiding visiting other places with birds and restricting visitors around your birds are helpful strategies to limit the spread of the disease. Before allowing anyone to enter your poultry premises, it is recommended to have them park their cars in a designated area away from your birds. It is always advisable to use a properly maintained footbath or wear designated cleaned footwear and/or clothing, and disinfect hands. Always clean and disinfect poultry equipment such as cages, crates, egg flat/racks, waterers, and feeders prior to reuse. APMV-1 is an enveloped RNA virus and is prone to most common disinfectants. The virus is inactivated by heating to 56 °C (133 °F) for 3 hours or 60 °C (140 °F) for 30 minutes. Acidic conditions with a pH ≤ 2 also destroy the virus. Like other viruses, APMV-1 can survive for long periods in cool conditions and in organic material such as feces.
Having known the signs of illness, report quickly to the nearest local state Department of Agriculture for follow-up to prevent future disease spread. The velogenic form of Newcastle disease should be reportable to the World Organization for Animal Health (OIE), which provides international guidelines for surveillance and eradication. Biosecurity information is also uploaded on the USDA and CDFA websites to provide education to poultry and pet bird owners to prevent disease.
Vaccination
Vaccines are available for chickens, turkeys, and pigeons and are used to induce an antibody response; therefore, vaccinated birds acquire disease only when they are exposed to large dose of NDV. Unfortunately, ND vaccines do not provide sterile immunity, and in many areas of the world, vaccines are used to prevent losses from sickness and death. Although there is an increase in number of reports of Newcastle disease outbreaks in vaccinated poultry, approved vaccines has done a lot to the cause of preventing clinical signs of disease under experimental conditions. Passively transferred maternal antibodies and antibodies from previous immunizations impact vaccination against NDV, and vaccination protocols with multiple deliveries (boosters) need to be carefully and properly designed. Field and environmental conditions, as well as concurrent infections also plays a great role in negatively impacting immune response to vaccination.
Live Virus Vaccines
Live lentogenic virus vaccines, chiefly B1 and LaSota strains, are most commonly used and typically administered to poultry by mass application in drinking water or by spray. Mucosal immunity induced in birds vaccinated by live virus vaccines applied by these routes decreases the amount of NDV the vaccinated birds will shed if infected with NDV, compared with the immune response induced by an inactivated virus vaccine. Mass vaccination methods require less labour. However, if they are not applied properly, they may lead to < 85% of the flock being immunized, the threshold needed for herd immunity. Alternatively, individually live virus vaccine is administered via the nares or conjunctival sac. Healthy chicks are often vaccinated at the 1st day of life. However, if vaccination is delayed till the second or third week, maternal antibody interference with an active immune response can be avoided.
Inactivated Virus Vaccines
After live virus vaccine, oil-adjuvanted inactivated virus vaccines can also be used in breeders and layers. In cases where live virus may be contraindicated (e.g., in pigeons), these vaccines can be used. In NDV endemic countries, a combination of live and inactivated virus vaccine can be used; alternatively, if permitted by law, a live mesogenic strain virus vaccine can also be used in older birds. Risk of exposure and virulence of the field virus challenge are the two factors that guide the frequency of revaccination to protect chickens throughout their lives. Administratiob of inactivated virus vaccines is more labor consuming, because each bird needs to be handled individually. Accidental inoculation of human tissues with oil-based vaccines requires prompt medical treatment.
Vectored Recombinant Vaccines
Fowl pox or turkey herpesvirus vectored NDV vaccines have the advantage of being able to be administered in ovo at the hatchery and are commercially available for chickens and have the advantage of being able to be administered in ovo at the hatchery. Reconstitution of these vaccines should strictly follow manufacturer’s guidelines. Because they take 3–4 weeks to produce a protective level of immunity, a window of exposure occurs during which chickens are susceptible to NDV infection and biosecurity becomes crucial.
Diagnosis
Confirmation of APMV-1/ ND via laboratory diagnosis is important because the virus can mimic many other poultry diseases such as avian influenza. So, the diagnostic tests may include:
- Real-Time Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR) – detects the presence of viral RNA from swab samples from oropharyngeal or cloacal swab samples.
2) Virus isolation (VI) – determines if viable virus is present by incubating embryonated eggs and determines virus type from swab samples.
3)Serology Blood Tests (i.e., Enzyme Linked Immunosorbent Assay (ELISA), Agar Gel Immunodiffusion (AGID), Hemagglutination Asssay (HA) / Hemagglutination Inhibition Assay (HI)) – identifies antibodies in blood serum; used as a screening test.
4) Differential diagnoses- Avian influenza, Avian Paramyxovirus, Infectious bronchitis, Infectious laryngotracheitis, Avian chlamydia, Mycoplasma, Avian cholera (pasteurellosis), Infectious coryza, acute poisoning (i.e., aflatoxin, insecticides, rodenticides), Egg drop syndrome (EDS 76), Duck plague (duck viral enteritis).
Reference
- Czegledi, D. Ujvari, E. Somogyi, E. Wehmann, O. Werner, B. Lomniczi
Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications.Virus Res., 120 (1–2) (2006), pp. 36-48
- de Leeuw, B. Peeters, Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae , J. Gen. Virol., 80 (Pt 1) (1999), pp. 131-136
- Huang, H.Q. Wan, H.Q. Liu, Y.T. Wu, X.F. LiuGenomic sequence of an isolate of Newcastle disease virus isolated from an outbreak in geese: a novel six nucleotide insertion in the non-coding region of the nucleoprotein gene. Brief report,Arch. Virol., 149 (7) (2004), pp. 1445-1457
- Krishnamurthy, S.K. Samal, Nucleotide sequences of the trailer, nucleocapsid protein gene and intergenic regions of Newcastle disease virus strain Beaudette C and completion of the entire genome sequenceJ. Gen. Virol., 79 (Pt 10) (1998), pp. 2419-2424
- Romer-Oberdorfer, E. Mundt, T. Mebatsion, U.J. Buchholz, T.C. MettenleiterGeneration of recombinant lentogenic Newcastle disease virus from cDNAJ. Gen. Virol., 80 (Pt 11) (1999), pp. 2987-2995
D.J. AlexanderNewcastle disease and other avian paramyxovirusesRev. Sci. Tech., 19 (2) (2000), pp. 443-462