One Health and Emerging Zoonotic Diseases: Infections Affecting Humans & Animals
Megha P. Kaore
Assistant Professor
Department of Veterinary Pathology
Nagpur Veterinary College
Nagpur 440 006 Maharashtra
Email id: meghakaore7@gmail.com
Abstract
One Health is a multidisciplinary concept aimed at improving human, animal, and environmental health. Emerging zoonotic diseases significantly impact human health, particularly those who live in poverty and have close contact with domestic or wild animals. Nearly 75% of zoonotic diseases are transmitted directly from animals to humans or indirectly via vector/agent interactions between animals and humans. Growing populations, globalization, urbanization, and the interaction of the environment with humans and livestock all play roles in the emergence and spread of zoonotic diseases.
Keywords: Emerging zoonotic diseases, one health, human, animal, environmental health
Introduction
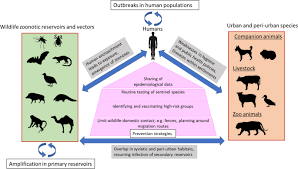
Not a single year passes without which we can tell the world: here is a new disease Rudolf Virchow, 1867. His saying is still valid in this present era where we have advanced our knowledge about diseases and develops advance diagnostics and technology but still emerging zoonotic diseases remain a crucial global challenge. In the 19th century, Robert Virchow coined the term “zoonosis” to describe pathogens that are naturally transmitted between vertebrate animals and humans. Later, during the 20th century, Calvin Schwabe revived the concept of “one medicine.” The one health approach plays a significant role in the prevention and control of zoonoses. Approximately 75% of new emerging human infectious diseases are defined as zoonotic. Approximately 60% are caused by multi host pathogens, characterized by their movement across various species. Zoonotic diseases are most commonly spread through direct contact from animals to humans or indirect contact via vector/agent interactions. India has the largest population, approximately 1.42 billion which shares common environment with total 303.76 million cattle, 148.89 million goats, and 9.06 million pigs and 851.81 poultry. In rural parts of India, animal lives in close proximity with humans. Interactions between humans, animals and environment provides opportunities for pathogens to spread.
Outbreaks of Severe acute respiratory syndrome (SARS), Nipah virus, highly pathogenic avian influenza viruses (HPAI), Middle East Respiratory Syndrome (MERS) coronavirus in the Gulf, and the Ebola virus in West Africa have augmented public awareness of the links between wild animals, livestock production, and global public health. Currently, One Health aims to develop the capacity and infrastructure to prevent and respond to the rapidly expanding zoonoses through research that is not only focused on the disease but also on the promotion of health at the individual, population, and ecosystem levels.
Economic burden of zoonotic diseases- In India
Large parts of the country have demonstrated to be global ‘‘hot spots’’ at high risk for emergence of pathogens from wildlife as well as domestic animals. In India, where approximately 80% of population lives-in rural areas in close contact with large domestic animal population. Abundance of vectors because of suitable climate, low socio-economic conditions and lack of proper medical care zoonotic diseases have great public health significance. However, because of inadequate diagnostic facilities, unfamiliarity of physicians with these diseases and lack of co-ordination between physicians, veterinarians, and epidemiologist, the extent of their existence is obscured. The plague outbreak occurred in September, 1994 incurring a total loss of over US$ US $ 1.7 billion (WHO, 2007). Brucellosis alone has contributed to loss of 30 million-man days and economic loss of Rs. 24 crores a year.
Factors responsible for emergence of zoonoses
Many factors contribute to the emergence of infectious diseases. Those frequently identified include microbial adaptation and change, human demographics and behavior, environmental changes, technology and economic development, breakdown in public health measures and surveillance, and international travel and commerce. New outbreaks of zoonotic disease continue to occur as the human-animal interface grows. Destruction of animal habitat and human population sprawl increases contact between humans and animals. Human can be exposed to new, unexpected zoonotic diseases or re-emerging previous diseases. Zoonoses that were previously limited to certain geographical locations can spread due to globalization of markets and increased worldwide travel.
Emerging Zoonotic Diseases
Bovine tuberculosis (bTB)
Most cases of human TB are caused by the bacterial species, Mycobacterium tuberculosis. Bovine tuberculosis is a chronic bacterial disease of animals caused by members of the Mycobacterium tuberculosis complex, primarily by Mycobacteriu. bovis. It is a major zoonotic disease, and cattle are the main source of infection for humans. It also affects other domesticated animals such as sheep, goats, equines, pigs, dogs and cats, and wildlife species such as wild boars, deer, and antelopes. Humans get infection by ingesting raw milk from infected cows, or through contact with infected tissues at abattoirs or butcheries.
- bovis which is estimated to account for up to 10% of human tuberculosis cases in developing countries. Infection of M. bovis is extrapulmonary and is naturally resistant to pyrazinamide, one of the antimicrobials that is commonly used to treat human tuberculosis. The WOAH, the World Health Organization (WHO), the Food and Agriculture Organization of the UN (FAO) and the International Union Against Tuberculosis and Lung Disease (The Union) jointly launched the first-ever roadmap to tackle zoonotic TB in October 2017. It is based on a One Health approach recognizing the interdependence of human and animal health sectors for addressing the major health and economic impacts of this disease.
Avian influenza: H5N1
H5N1 has killed millions of poultry in a growing number of countries throughout Asia, Europe and Africa. Health experts are concerned that the coexistence of human flu viruses and avian flu viruses (especially H5N1) will provide an opportunity for genetic material to be exchanged between species-specific viruses, possibly creating a new virulent influenza strain that is easily transmissible and lethal to humans. The mortality rate for humans with H5N1 is 60%. From 2003 to 25 March 2024, a total of 888 worldwide human cases of infection of influenza A(H5N1), including 463 deaths, have been reported to WHO from 23 countries. On 21 July 2021 India notified first human case of avian Influenza A(H5N1) from Haryana state, northern India.
Nipah Virus
Nipah virus (NiV) infection is an emerging zoonotic disease of public health importance in the South-East Asia Region with a high case fatality rate estimated to range between 40 and 75%. The 2018 Kerala Nipah virus outbreak traced to the fruit bats in the area. The outbreak was localized in Kozhikode and Malappuram districts of Kerala and claimed 17 lives. Between 12 and 15 September 2023, a total of six laboratory-confirmed cases of Nipah virus infection including two deaths were reported by the State Government of Kerala. Two previous outbreaks occurred in the state of West Bengal in 2001 and 2007.
Severe acute respiratory syndrome (SARS)
It is first Emerging Infectious disease of the 21st Century. It is a viral respiratory disease of zoonotic origin caused by the SARS coronavirus (SARS-CoV). Bats and civet cats are considered as reservoir. Between November 2002 and July 2003, an outbreak of SARS in southern China caused an eventual 8,098 cases, resulting in 774 deaths reported in 37 countries.
Middle East respiratory syndrome coronavirus (MERS-CoV)
Middle East respiratory syndrome (MERS) is a viral respiratory disease caused by Middle East respiratory syndrome coronavirus (MERS‐CoV) that was first identified in Saudi Arabia in 2012. MERS-CoV is a zoonotic virus and has been identified and linked to human infections in dromedary camels in several Member States in the Middle East, Africa and South Asia (WHO).
Scrub typhus
Scrub typhus, a rickettsial infection caused by Orientia tsutsugamushi, a gram-negative obligate intracellular coccobacillus is transmitted to humans by the bite of larval stage (chigger) of trombiculid mite. The disease has been reported from all over the world, but it is endemic in
terrains of the tsutsugamushi triangle, a geographical region comprising of South and East Asia and the Southwest Pacific. Recent reports from India suggest that there is a resurgence of scrub typhus and that the resurgence is associated with considerable morbidity and mortality. This is a cause of concern. Though considered as disease of rural areas, this disease has been urbanized and the prevalence has broadened further. Increasing prevalence of scrub typhus may be attributed to combination of climate change and expansion of humans into previously uninhabited areas and widespread use of Beta-lactam Antibiotics. India, no definite statistics available due to lack of awareness, high cost of diagnostic kits and the fact that it is not a reportable illness (Viswanathan et al., 2013).
Rabies:
Rabies is known to be present in more than 150 countries and territories of all continents except Antarctica. About 60 000 people die of rabies every year, mostly in Asia and Africa. Rabies virus infects domestic and wild animals and is spread to people through close contact with infected animals’ saliva via bites or scratches. The main route of rabies transmission to humans is the bite of rabid dogs.
Tick Borne Zoonoses
It includes: Lyme Borreliosis, Crimean – Congo Haemorrhagic Fever, Kyasanur forest disease.
Crimean-Congo haemorrhagic fever
CCHF virus is a member of the genus Nairovirus of the family Bunyaviridae. Ixodid ticks of the genus Hyalomma specifically act as a vector and reservoir for the virus and numerous wild and domestic animals can serve as amplifying hosts. Asymptomatic in infected animals but highly fatal in humans. During the past decade, the virus has emerged in new areas of Europe, Africa, Middle East, and Asia and has increased in disease endemic areas (Leblebicioglu et al., 2010). In India on January 2011, the nosocomial outbreak of CCHF has been reported from Gujrat, India (Makwana et al., 2015). Since then, numerous outbreaks and sporadic cases of this disease have been reported from different districts of Gujarat State.
Kyasanur Forest Disease (KFD)
It is viral haemorrhagic disease transmitted through bite of infected ticks. KFD virus is an enzootic in India and maintained in ticks, mammals, and birds. KFD was first recognized in 1957 in the Kyasanur Forest of Shimoga District, Karnataka State, India. More than 3,314 monkey deaths attributed to KFD were reported in KFD-endemic states in India during 1957–2020 (Chakraborty et al., 2021). People with recreational or occupational exposure to rural or outdoor settings (e.g., hunters, herders, forest workers, farmers) within Karnataka State are potentially at risk for infection by contact with infected ticks. Its geographical distribution now expands to Tamilnadu and Kerala states. About 22.4 percent of persons living in the Andaman and Nicobar Islands were found to be seropositive for KFD in 2002; and human infection by closely related agents is reported in Saudi Arabia (Alkhurma virus) and China (Nanjianyin virus). Since January 2024, two people have died due to Kyasanur Forest Disease (KFD) in Karnataka.
Japanese encephalitis
Japanese encephalitis is a mosquito-borne viral disease that can cause reproductive losses in pigs, and encephalitis in horses. Japanese Encephalitis Virus (JEV) is the main cause of viral encephalitis in many countries of Asia with an estimated 68, 000 clinical cases every year. Case-fatality rate among those with encephalitis can be as high as 30%. Permanent neurologic or psychiatric sequelae can occur in 30%–50% of those with encephalitis. Seasonal outbreaks: JE cases in India often follow a seasonal pattern, with increased transmission during the monsoon and post-monsoon periods when mosquito populations are higher. Outbreaks typically occur from May to October, peaking during the rainy season.
Q fever
It is an emerging occupational and foodborne zoonoses caused by the bacteria Coxiella burnetii which can infect mammals, birds, reptiles and arthropods. Veterinarians, laboratory workers, farmers and abattoir workers at risk. It can cause abortions and still births in cattle, sheep, and goats. It is an endemic in many countries including India. It is spread by close animal contact and common housing. Q fever can also be spread by ticks which pass the bacteria. Since it is also shed in the milk of an infected animal, it can be contracted by drinking non-pasteurized infected milk. In Netherland it forced Culling of 55,000 goats in order to control Outbreak in Humans (Nature, 2010, 2nd Highest in the world history following BSE episode). It has been reported from Punjab, Haryana, Rajasthan, UP, M.P. & Karnataka (Vaidya et al., 2010). In 2023 Hyderabad-based National Research Centre on Meat confirmed through serological tests that five butchers among 250 samples.
Leptospirosis
Leptospirosis are important zoonotic disease that can be transmitted to humans. In the wild, the disease occurs in animals, mostly rodents (rats and mice), dogs, cattle, goats and wild boars. In the past decade, leptospirosis has emerged as a globally important infectious disease. It occurs in urban environments of industrialized and developing countries, as well as in rural regions worldwide. Mortality remains significant, related both to delays in diagnosis due to lack of infrastructure and adequate clinical suspicion, and to other poorly understood reasons that may include inherent pathogenicity of some leptospiral strains or genetically determined host immunopathological responses.
Anthrax
The disease anthrax occurs generally in herbivores and the causative organism (Bacillus anthracis) infects humans who come in contact with infected animals or their products. A recent study mapping the distribution of anthrax across 70 countries estimated that approximately 1.83 billion people lived within the regions of anthrax risk globally, mostly concentrated in rural rainfed systems throughout arid and temperate land across Asia, southern Europe, sub-Sahelian Africa, North America and parts of Australia (Carlson et al., 2019). India is an endemic country for animal anthrax which leads to sporadic and seasonal outbreaks in humans. The case fatality rate of cutaneous anthrax ranges from 2% to 38%, while the gastrointestinal and inhalational types are usually 100% fatal. Poverty, socio-cultural practices & environmental factors exacerbated the risk.
Echinococcosis
Echinococcosis is a serious zoonosis, with rates of human cystic echinococcosis infection ranging from less than 1 per 100,000 to more than 200 per 100,000 in certain rural populations where there is close contact with domestic dogs. A total annual loss of Rs. 11.47 billion in India is estimated with cattle and buffalo population. Laboratory workers, animal handlers, veterinarians, dog owners are all at higher risk of infection. Since the eggs are shed in the environment, they can contaminate fruits, vegetables or water, or can stick to the fur of an animal and be transferred on hands to the mouth.
Control and prevention of zoonotic diseases
Early detection of zoonotic pathogens through enhanced laboratory capacity and surveillance at the animal–human interface is a crucial step toward controlling and preventing zoonoses. Rapidly detecting, responding to, and controlling public health emergencies at their source, including those caused by outbreaks of zoonotic diseases, is essential for global health security. In India, there is an active effort to strengthen surveillance for early diagnosis and effective, timely containment. The National Centre for Disease Control plays an important role in strengthening capacity across the country and bringing together epidemiologists, microbiologists, veterinarians, entomologists, etc., to effectively launch required multi-sectorial action to address zoonotic diseases. Under Niche Area of Excellence on Centre for zoonoses the Maharashtra Animal and Fishery Sciences University, Nagpur in collaboration with ICAR-ICMR has been established as a unique platform for veterinary and human medicine to combat zoonotic disease to achieve One Health approach. National Institute for One Health (NIOH) established in will further expedite the country’s efforts in enhancing health research to serve our vulnerable populations.
References:
Viswanathan S, Muthu V, Iqbal N, Remalayam B, George T. Scrub typhus meningitis in South India—a retrospective study. PLoS one. 2013 Jun 14;8(6):e66595.
Leblebicioglu H. Crimean–Congo haemorrhagic fever in Eurasia. International journal of antimicrobial agents. 2010 Nov 1;36:S43-6.
Makwana D, Yadav PD, Kelaiya A, Mourya DT. First confirmed case of Crimean-Congo haemorrhagic fever from Sirohi district in Rajasthan State, India. Indian Journal of Medical Research. 2015 Oct 1;142(4):489-91.
Chakraborty S, Sander WE, Allan BF, Andrade FC. Retrospective study of Kyasanur forest disease and deaths among nonhuman primates, India, 1957–2020. Emerging infectious diseases. 2021;27(7):1969.
Carlson CJ, Kracalik IT, Ross N, Alexander KA, Hugh-Jones ME, Fegan M, Elkin BT, Epp T, Shury TK, Zhang W, Bagirova M. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nature microbiology. 2019;4(8):1337-43.
Vaidya VM, Malik SV, Bhilegaonkar KN, Rathore RS, Kaur S, Barbuddhe S. Prevalence of Q fever in domestic animals with reproductive disorders. Comparative immunology, microbiology and infectious diseases. 2010 Jul 1;33(4):307-21.
https://www.who.int/news-room/fact-sheets/detail/echinococcosis