One Health- One World : Prevent Zoonoses
M.Nithya Quintoil* and A. Varun
*Corresponding author:
M.Nithya Quintoil
Assistant Professor
Department of Veterinary Public Health,
Rajiv Gandhi Institute of Veterinary Education and Research,
Kurumbapet, Puducherry-605009
Email: m.n.q.mohandasse@gmail.com
Tel: +91 8220409141
Abstract
Progress in science and research facilitated disciplines and areas of expertise with specialized institutes, journals, funding, and even language. At global context, the broad disciplines of Human medicine, Veterinary medicine and Dentistry evolved, with the swift in the development of many specialized areas of human medicine with discipline-specific information, skills, qualifications, and outcomes that were required, enabling real progress to be made using targeted resources to address specific problems with a solution. But, over time mankind has faced emergence of zoonotic diseases with pandemic potential which has its origin in animals. In this context to safeguard human and animal health, researchers throughout the world were thoughtful for devising state-of-the-art understandings that can come from a comparative approach with the application of learnings from one discipline across to another through a multidisciplinary, multisectoral approach with the emergence of “One Health” Concept. Globally it is most acknowledged approach with collaboration from various distinct sectors of agriculture, wildlife biology, and human health and, in many countries, One Health approach has been adopted by the Government and in research, policy development, and field activities. Zoonotic diseases are on increasing trend globally as well as in India. Current developments in diagnostics have implied that there are about 1407 human pathogens with the potential to cause disease of which 816 were zoonotic, i.e., capable of being transmitted naturally between animals and humans. These include 538 bacteria and rickettsia, 317 fungi, 208 viruses, 287 helminths, and 57 protozoa. The impact by environmental and climatic changes; with increase in international commerce; rapid global transport; recreational activities in forest; wild animal trade; bush meat; human and animal population dynamics, and emerging drug resistance among vectors and pathogens are responsible for the increase in zoonotic diseases. The Concept of one health, particularly in developed countries is slowly established, and these nations take the advantages and benefits of this approach in tackling zoonoses are diverse, on the other hand developing countries where zoonoses have the greatest impact the concept is still not outwardly implemented. In recent past, international organisations like World Health Organization, Food and Agriculture Organization, and the World Organization for Animal Health have collectively called for a One Health approach to better understand the problem as well was to develop solutions for the emergence of new Zoonotic diseases at different situations. With this background, this review article details elaborately the various facets of One health and zoonotic diseases.
Keywords: One Health, Zoonosis, Human, Environment, Animals,
Graphical abstract
Introduction
The emergence and outbreak of severe acute respiratory disease (SARS), the first severe and readily transmissible novel disease, led to the realisation that a formerly unidentified pathogen could emerge from a wildlife source at any time and in any place and, without warning can threaten the health, well-being, and economies of all societies, further there was a clear need for countries to have the capability and capacity to maintain an effective alert and response system to detect and quickly react to outbreaks of international concern, and to share information about such outbreaks rapidly and transparently. Similarly, the emergence and spread of influenza H5N1 has been another excellent example which demanded the importance of global cooperation. (Mackenzie et al., 2014). During the year 2004, Wildlife Conservation Society devised a series of strategic goals known as the ‘Manhattan Principles’ which clearly pointed out the link between human and animal health and the threats imposed by these diseases on food supplies and economic implication in a community were well addressed. In addition to this, these principles were basis for recognising the critical importance of collaborative, cross-disciplinary approaches for responding to emerging and re-emerging diseases, and in particular emphasized the importance of including wildlife health as an essential component of global disease prevention, surveillance, control, and mitigation. In this context a response for large multi-national outbreaks or pandemics requires global cooperation and participation has led to the evolution of the term ‘One Health’ during the year 2003–2004.
Genesis of One Health
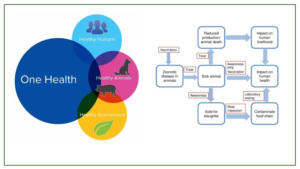
The concept of One Health is not new and can be traced back for at least two hundred years (Atlas, 2013), firstly as One Medicine, but then as One World-One Health and eventually One Health. There is no single, internationally agreed upon definition of One Health, although several have been suggested. The most commonly used definition by the US Centers for Disease Control and Prevention and the One Health Commission is: ‘One Health is defined as a collaborative, multisectoral, and transdisciplinary approach—working at the local, regional, national, and global levels—with the goal of achieving optimal health outcomes recognizing the interconnection between people, animals, plants, and their shared environment’. A definition suggested by the One Health Global Network is: ‘One Health recognizes that the health of humans, animals and ecosystems are interconnected. It involves applying a coordinated, collaborative, multidisciplinary and cross-sectoral approach to address potential or existing risks that originate at the animal-human-ecosystems interface’. A much simpler version of these two definitions is provided by the One Health Institute of the University of California at Davis: ‘One Health is an approach to ensure the well-being of people, animals and the environment through collaborative problem solving—locally, nationally, and globally’. The genesis of One health approach is depicted in Fig1
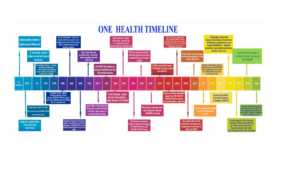
Figure 1: Chronicle of One Health
Multidisciplinary approach -One health:
The One Health concept clearly focusses on consequences, responses, and actions at the animal–human–ecosystems interfaces, and especially (a) emerging and endemic zoonoses, the latter being responsible for a much greater burden of disease in the developing world, with a major societal impact in resource-poor settings (Welburn., et al 2015; Cleaveland., et al 2017) antimicrobial resistance (AMR), as resistance can arise in humans, animals, or the environment, and may spread from one to the other, and from one country to another (Hoelzer., et al 2017 ); and food safety (Boqvist., et al 2018; Garcia., et al 2018 ). However, the scope of One Health as envisaged by the international organizations (WHO, FAO, OIE, UNICEF), the World Bank, and many national organisations also clearly embraces other disciplines and domains, including environmental and ecosystem health, social sciences, ecology, wildlife, land use, and biodiversity. Engaging the medical community more fully in the future may require the incorporation of the One Health concept into the medical school curricula so that medical students see it as an essential component in the context of public health and infectious diseases (Rabinowitz., et al 2017). One recent development that might help in generating increased global awareness of the One Health concept, particularly among students, but also more generally, has been the designation of November 3rd as One Health Day, Initiated in 2016 by the One Health Commission (www.onehealthcommission.org). One Health Day is celebrated through One Health educational and awareness events held around the world. Students are especially encouraged to envision and implement One Health projects, and to enter them into an annual competition for the best student-led initiatives in each of four global regions. Today’s health problems are frequently complex, transboundary, multifactorial, and across species, and if approached from a purely medical, veterinary, or ecological standpoint, it is unlikely that sustainable mitigation strategies will be produced. One Health approach for understanding and mitigating many current complex health problems involves innovative approaches and outcomes but the range and types of collaborative partnerships required are multidisciplinary in nature. The multidisciplinary rationale approach of one health is depicted in Fig.2.
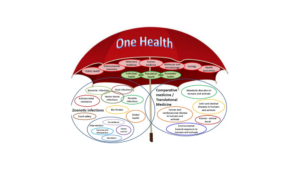
Figure 2: One Health: A multidisciplinary approach
One Health in India
| 2007 | :Indian Prime Minister’s Address at the International Ministerial Conference on Avian and Pandemic Influenza: PM uttered “In this specific case of Avian flu, we need to focus as much on human health as on animal health. Investments in public health will be unproductive without ensuring the health of our livestock. |
| 2009 | :OHASA was founded by the Wildlife Trust to bring together scientists and policymakers from India’s Ministries of Health, Agriculture, and Environment, along with NGOs & universities from India, Bangladesh, Pakistan and Nepal, to combat zoonotic illnesses. |
| 2010 | :The National Center for Disease Control and Prevention (NCDC) has established a Global Disease Detection (GDD) – India Centre in response to the need to expand collaboration on health between India and the United States, particularly in the area of emerging global diseases. The Ministry of Health and Family Welfare (MOHFW), the Government of India (represented by NCDC), and the US Department of Health and Human Service, represented by the Center for Disease Control and Prevention (CDC), have partnered on this project. In addition, the NCDC also administers a programme called Inter-sectoral Coordination for Prevention and Control of Zoonotic Disease, which aims to improve inter-sectoral coordination between the medical, veterinary, and wildlife sectors, as well as other relevant stakeholders |
| 2014 | :On February 26th, 2014, the Kerala Veterinary and Animal Science University launched a Centre for One Health Education, 36 Advocacy, Research, and Training (COHEART), as well as two new One Health courses: a PG Diploma in One Health and a PG Certificate in One Health Surveillance. COHEART has been involved in several One Health initiatives since its establishment |
| 2016 | :The Veterinary Council of India has integrated the One Health concept in the BVSc curriculum for the first time under Unit 1- Veterinary Public Health and Food Safety, in accordance with the authority provided by the Indian Veterinary Council Act, 1984. |
| 2017 | :India’s National Action Plan on Antimicrobial Resistance incorporated the One Health Approach |
| 2019 | :One Health India Declaration: During the conference held in New Delhi, the decision was made by the scientific community to launch India’s One Health Initiative to tackle the most urgent health threats and have also proposed a One Health roadmap for India. The conference was hosted by the Department of Biotechnology (DBT), Ministry of Science and Technology in partnership with the Ministry of Agriculture & Farmers’ Welfare and Ministry of Health & Family Welfare and their respective departments
Delhi Declaration Signed by Health Minister of India and the other Member States to Advocate the ‘One Health’ Approach: |
| 2020 | : Launch of One Health Centre in Maharashtra: The Maharashtra Animal and Fishery Sciences University and the ICMR-National Institute of Virology in Pune partnered together to establish the OH centre.
: Proposed National Institutional Platform for One Health: The Indian Council of Medical Research will contribute to constructing a national institutional platform for One Health which will boost research initiatives, according to the central government, which announced recovery plans for the COVID-19 crisis as part of the Atma Nirbhar Bharat economic stimulus package. |
| 2021 | :The Health Minister announced the formation of a high-level steering committee for India’s eco-health projects that will have its secretariat at the ICMR and be backed by the planned National Institute for One Health. |
Zoonoses
The word zoonoses originates from the Greek words ‘zoo’ (animal) and ‘noses’ (disease). Rudolf Virchow coined the term zoonosis in 1885. ‘Those diseases and infections which are naturally transmitted between vertebrate animals and man,’ according to the Joint Expert Committee of WHO and FAO (1959). Many zoonotic diseases are associated with managing infected wild and domestic animals from their natural environment or farms to markets. Out of the 1405 infectious illnesses, 61 percent are zoonotic. In addition, 73 percent of all newly found infectious diseases, often known as emerging infections, have been classified as zoonotic over the last three decades (Gregar, 2007). Those who work with animals (laboratory researchers, farmers, veterinarians, abattoir workers, zoo workers, wool classers, pet shop operators) or possess animals as pets at home risk developing a zoonotic disease. India has a significant livestock and poultry resource that helps improve the rural population’s social-economic conditions. Recent nipah and avian influenza outbreaks in India have once again demonstrated the ability of microorganisms from animal hosts to adapt to human hosts. Animal health and productivity are intrinsically related to human health. This bond between humans and animals, as well as the environment, is especially strong in poor countries, as animals offer transportation, draught power, fuel, and clothes, as well as sources of protein (milk, egg and meat). Zoonoses are diseases spread by ill domestic and wild animals transported from their native habitats or farms to markets, slaughtering slabs and abattoirs. Because of the extraordinarily rapid rate of human society’s growth, which has transformed the entire world into a global village, the threat of zoonoses has grown with each passing day.
Factors responsible for occurrence of Zoonoses
Unsanitary conditions and overpopulation : Slums have a higher risk of illness due to unsanitary conditions and overpopulation. Furthermore, increased human animal population density and interaction are key elements in spreading bovine tuberculosis. Similarly, an inefficient waste disposal system has been linked to increased leptospirosis cases.
Urbanization: Rapid urbanization raises disease and health burdens while also bringing epidemiological and nutritional challenges. Depending on the production processes and market structures of live food animals, urban inhabitants are often less vulnerable to animal contact than rural populations. Still, urbanites may live in more congested settings conducive to disease transmission.
Human Deeds and Food Habits: The global need for animal protein is rising, with shocking forecasts for consumption. The majority of this demand is predicted to arise from the developing world. Growing populations and rising per-capita incomes will force people to switch from a rice, bean, and corn-based diet to one that includes more animal protein, a concept called “nutrition changeover”. (ARÁMBULO and Thakur,1992)
Population Growth: The chance of animal-human connection grows as more people are added to limited area. Viruses that are transmitted from animals to humans are becoming more common as the human population grows, intruding on wildlife habitats and destroying ecosystems. Furthermore, population increase will continue to fuel deforestation, loss of habitat, and climate change, which exacerbate the danger of zoonoses outbreaks.
Anthropological and Cultural Factors: Uncontrolled wildlife trade, increased traffic in animal products, increased agriculture, habitat loss, deforestation and other land-use changes, antibiotic resistance, and climate change are the result of human activity most likely to put people at risk of developing zoonoses. Human-caused ecological and environmental changes, such as dam construction, result in losing natural habitats for free-ranging animals. Another definition of bioterrorism is the deliberate distribution and use of microorganisms to endanger a country’s security. (Singh etal.,2011)
Travel: Once a zoonotic illness has been established, population movements are likely to aid its spread among the human population. When visiting tropical regions, travellers (tourists, entrepreneurs, and other professionals) are not only at risk of catching communicable diseases, but they can also act as vectors for transmitting infectious diseases to another region or even the entire globe, as in the case of Covid-19.
Natural Disasters: Plague outbreaks are frequently linked to natural calamities. Displacement of people, a lack of preparation, sanitation, safe drinking water, and primary healthcare centers are all factors that contribute to zoonotic disease outbreaks after disasters.
Global Warming: The rise of various vector-borne (e.g., yellow fever, malaria, leishmaniasis, dengue fever) and water-borne (e.g., cholera) diseases is favoured by climate.
Pathogen Adaptation: Zoonosis is causing an increase in mortality and morbidity indicates that diseases have already crossed the host-species barrier. Phylogenetically similar hosts (anthroponosis) are more prone to pathogen adaptation and transmission.
Transmission of Zoonotic Disease
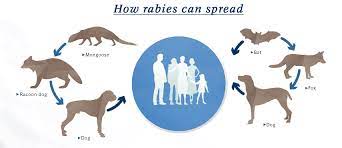
Zoonoses can be transmitted in various ways, from the source of infection to a susceptible host (Fig 2). However, the primary means of transmission are divided into two categories: Direct Transmission: Certain diseases, such as leptospirosis, pox, and dermatophytosis, are transmitted by direct contact between the source of infection and the vulnerable host. Droplet infection is another way for diseases to spread. Droplets of saliva or nasopharyngeal secretions are projected directly onto other hosts’ conjunctiva or mucous membranes. Tuberculosis, the common cold, and diphtheria, for example, are commonly observed when sneezing, coughing, or talking. Disease transmission can also occur when susceptible tissues are exposed to disease agents in soil or decaying matter, such as hookworm infection and tetanus. Direct transmission can also happen through an animal bite (rabies) or transplacental/vertical transmission from one generation to the next (toxoplasmosis). Indirect Transmission: Transmission by vector-borne (arthropod) vectors, either mechanically or biologically. Mechanical transmission occurs when an infectious agent, such as serum hepatitis, amoebiasis, or cholera, does not grow or multiply on or within the vector. Biological transmission happens when an infectious agent grows and multiplies in a vector and requires a period of incubation before transmitting the disease. It could be the Propagative Type (where the agent only breeds in the vector but does not grow, such as plague bacilli in rat fleas) or Cyclo-propagative type (when the agent grows and multiplies in the vector, such as malaria parasites in mosquitoes). In addition, the agent could be cyclo developmental (just development, e.g. microfilaria in mosquitoes) or transovarian (spread from one generation to another, e.g. tick-borne encephalitis). Water, food (including milk, meat, fish, vegetables and fruits), blood, serum, and other biological products such as organs and tissues are numerous means 49 of indirect vehicle-borne transmission. Infectious agents can be conveyed through a vehicle biologically or mechanically, such as hepatitis A virus in water, salmonellosis, Trichinella spiralis in meat, tuberculosis in milk, brucellosis in fish, and Vibrio parahemolyticus in fish. Indirect transmission takes place through the air via two routes: droplet nuclei and dust, as well as dirty hands and fingers (e.g., streptococcosis, staphylococcosis, colibacillosis, salmonellosis)
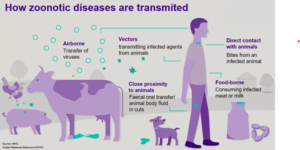
Figure 2: Transmission of zoonotic diseases
Impact of Zoonotic Disease
Monetary Impact: The economic consequences of zoonotic illness can be far-reaching, affecting everything from local farmers’ markets to international trade agreements. The first step in developing measures to avoid the spread of zoonoses, the restoration of trade, and the continuation of services following an outbreak is to understand these effects. For example, SARS, a virus that causes acute respiratory disease, is thought to have developed in China in 2002 and 2003 when it is believed to have taken a zoonotic leap. Within months, this infectious sickness had spread from Asia to North and South America and Europe. SARS afflicted 8,098 people until it was finally eradicated, with nearly 800 of them dying. SARS has a significant financial impact on many more people around the world. It had a measurable effect on Asian Countries’ and Canada’s GDPs. Tourism, trade and retail were among the businesses that suffered losses totalling $50 billion globally. (Cascio et al.,2011)
Impact on Employment: Zoonoses can have various effects on worldwide employment. Companies may see lower attendance due to infection, the fear of infection, or workers caring for their families. Reduced workforces may generate a broader economic problem, resulting in an economic slump and increased unemployment.
Impact on Economy and Global Trade: Zoonoses outbreaks in animals pose a significant threat to farming. Previous crises or fears related to food supply contamination have eroded customer confidence, resulting in abrupt and substantial reductions in the consumption of the affected products and price reductions. In addition, culling diseased cattle, dropping animal value from control measures like mandatory emergency vaccination, and costs from business interruptions can all result in losses. (Marano et al., 2007)
Impact on Transport, Travel, Tourism, and Social Gatherings: Even a modest zoonoses epidemic in a country can quickly spread throughout the international society thanks to contemporary technology. Visitors to that country may sense a threat and be concerned about their personal safety. Commuters and tourists cancelling visits or fleeing the affected country can significantly influence the economies of the affected countries. In addition, for public health considerations, social events such as symposia and congresses may be cancelled. (Keusch etal., 2009)
Impact on Delivery of Health Care: The concern of a pandemic, or even a catastrophic epidemic like SARS, having a significant and potentially severe effect on the healthcare industry is growing. While dealing with significant supply restrictions, health care professionals are thinking and planning how to cope with an unforeseen number of patients in emergency rooms and hospitals. In addition, employees on the front lines of infectious exposure may be further harmed since they must handle many patients and unaffected people seeking medical assurance.
Miscellaneous effects:
- High morbidity will result in acute and long-term debilitating diseases
- An increase in the rate of mortality in animal and human populations (Meslin,2006)
- Morbidity has a negative effect on production and reproduction
- Infection-related impairments in animal output
- Medical expenses for healthcare include hospitalization, diagnostic testing, and other lab services
- Loss of man-hours leads to a loss of earnings from infirmity
- Negative influence on employee morale, as in the case of tuberculosis, requires lengthy treatment
- Malnutrition decreases a large number of people’s resistance
- Diseases that cause havoc on animal production may cause many nations to import meat, milk, leather, wool, and other animal goods. This depletes the foreign exchange required for development.
- Unfavourable publicity and socio-economic losses affect the mental status of affected human beings
- Medico-legal implications
Wildlife-Spillover of Zoonotic Diseases:
A number of wild animals acts as Carriers of Disease (Thompson,2013). It’s crucial to identify the emergence of new viruses if we want to prevent their spread humans. A recent study by EcoHealth Alliance, compiled data on viruses that infect mammals, about 600 viruses were identified in over 750 species. They were then able to quantify the number of viruses in each species and find traits that indicate a high likelihood of human transmission. Living in close proximity to humans and being genetically connected to them increases the chances of transmission. Bats have the largest number of these viruses of any species studied. The Mammalia, Reptilia, Aves, and Amphibia groups have the most potential risk of zoonotic disease spillover. The number of viruses that mammalian species have transmitted to humans was counted to investigate zoonotic viral spillovers. It was discovered that the number of zoonotic viruses detected in mammalian species is in proportion to the abundance of global species. This suggests that the virus transmission risk has been the highest from animal species that have increased in abundance, especially those that have expanded their home range by adapting to human-dominated landscapes. Bats, primates and domesticated species were identified as having more zoonotic viruses than other species. In addition, threatened wildlife species with population declines due to exploitation and habitat loss transmitted more viruses with people. Hunting and trade of wildlife facilitate close contact between wildlife and humans, and the findings add to the growing body of evidence that exploitation and anthropogenic activities have resulted in habitat degradation, enhanced opportunities for animal-human interactions and enabled zoonotic disease transmission (Christine K. Johnson et al., 08 April 2020). Structured variation among mammalian species has implicated them as a possible source of virus spillover to humans. This finding was revealed by a global-scale analysis across the breadth of all zoonotic viruses, with specific patterns in zoonotic virus richness related to the domestication of species and recent trends in wildlife populations. The orders Rodentia (61 percent), Chiroptera (30 percent), Primates (23 percent), Artiodactyla (21 percent), and Carnivora (18 percent) were found to have the highest proportion of zoonotic viruses, while other mammalian orders had fewer viruses. It was also discovered that three mammalian orders (rodents, bats, and primates) had been implicated as hosts for the bulk of zoonotic viruses known to date (75.8%), and these orders account for 72.7 percent of all terrestrial mammal species (Christine K. Johnson, et al., 08 April 2020).
Disease Outbreaks Linked to Wildlife Trade or Spillover
- HIV spread from chimps and other non-human primates to humans through hunting and slaughtering, exposing people to animal bodily fluids. (Glaser etal., 1994)
- The Nipah virus spread from fruit bats to pigs and ultimately to humans in Malaysia due to intensive pig farming and the existence of orchards on farms that attract bats and allow pigs to eat bat-contaminated fruit dropped by bats.
- The SARS coronavirus originated in southern China through the wildlife trade. Live civets, bats, and other mammals were smuggled into big urban markets and killed, exposing sellers to sick animals’ bodily fluids. Civet vendors, in particular, were infected at a significant rate, and the virus was isolated from civets.
- From 2003 through 2015, avian influenza H5N1 was first found in hawk eagles trafficked into Belgium from Thailand, resulting in 826 human infections and 440 deaths.
- Monkeypox was first discovered in a wild mouse imported from Ghana for the pet trade in 2003.
- Ebola Virus in 2013-2016: The virus was first found in West Africa through the sale of bats and non-human primates, and it resulted in over 11,310 human cases and fatalities.
- The current new coronavirus (COVID-19) outbreak is linked to a live animal marketplace in Wuhan, China, and is believed to have been originated from Bats. SARS-CoV-2 can be transmitted to mink, cats, ferrets, and non-human primates. (Naveenkumar etal., 2020)
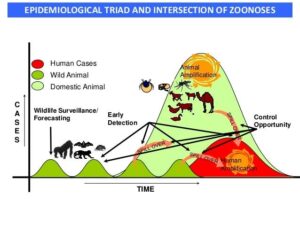
Figure 3: Epidemiological triad and intersection of zoonoses. (Karesh et al., 2012)
Control of Zoonoses
The following are the various control measures which are detailed below (Kumar etal., 2015)
Quarantine: Limiting the mobility of people or animals who have been exposed to a contagious disease for a duration of time not exceeding the disease’s customary incubation period in place to avert effective
contact with individuals who have not been exposed. It is a preventive step, including the separation of healthy people from others who appear to be healthy but are suspected of being infected. A quarantine
period of 14 days, or 90 days or more in some situations, is deemed acceptable. Animals in exhibits, especially carnivores and omnivores, should be isolated for 180 days. Imported dogs should be confined
for four (anti-rabies vaccinated dogs) or six months (anti-rabies unvaccinated dogs), with one dose of
rabies vaccination administered on the 90th day of quarantine.
Slaughter: Infected animals may have to be killed during eradication programmes to eliminate infection
sources. A ‘test-and-removal’ technique is used to eradicate specific illnesses from herds, in which all
animals are tested, and only those that test positive are removed and murdered. Pre-emptive slaughter:
During epidemics, the culling of infected animals is frequently accompanied by the slaughter of animals
who may have been exposed to infection and are thus at risk of disease, such as those who have directly
come into contact with people and vehicles that have been exposed to infected animals. For example,
avian influenza. Contiguous culling (the blanket killing of animals on the premises immediately around an infected farm) is a type of pre-emptive killing that seeks to ‘get ahead’ of the infection before clinical
indications appear. In epidemics, animal slaughter is often followed by various treatments to reduce the
danger of transmission (e.g., disinfection and carcass destruction by burning or burial); this is referred to
as ‘stamping out.’
Vaccination: Vaccines can protect against a variety of germs and viruses, as well as some helminths. Vaccines can be inactivated (for example, by alkylating chemicals, which prevent bacterial and viral nucleic acid from replicating) or live, with the organisms usually attenuated. Each vaccine has its own set of benefits and drawbacks. Inactivated vaccines are better than live vaccines when novel agents or serotypes are found and may be made more quickly. However, they are more expensive than live vaccines and induce mucosal and cell-mediated immunity less rapidly and effectively, necessitating periodic ‘booster’ doses. However, there is a risk that the live immunizing agent will return to a virulent state. It may also be difficult to distinguish between infections caused by live vaccinal strains of the agent and illnesses caused by natural ‘field’ strains (e.g., foot-and-mouth disease).
Therapeutic and Prophylactic Chemotherapy: Antibiotics, anthelmintics, other medications, and hyperimmune serum are used to cure diseases (therapeutically) and prevent disease (prophylactically) at periods of high risk to boost production. Antibiotics are used preoperatively and postoperatively to avert bacterial infections, and antibiotics are added to livestock feed to encourage growth (a declining practice).
However, these processes can result in unfavourable outcomes, such as selecting bacteria strains immune
to the antibiotic they were treated. Bacterial resistance can also be disseminated by transferring it to other bacteria.
Movement of Hosts: Animals can be evacuated from endemic illnesses in ‘high risk’ locations. This management method is used in tropical nations where hosts migrate from places where biological vectors
are active on a seasonal basis. For example, in the wet season, the Fulani tribe in West Africa customarily migrates from the south to the north with their livestock to avoid the tsetse fly. Horses may also be brought indoors at night to prevent infection from the African horse disease virus, which is spread by night-flying Culicoides vectors.
Restriction of Movement of Hosts: During epidemics and eradication programmes, animal mobility is
frequently restricted to reduce the danger of disease transmission. Other strategies aimed at lowering the
likelihood of disease spread inside zones, such as slurry control and hunting, are also being considered.
Mixed, Alternate and Sequential Grazing: Mixed, alternating, and sequential grazing can minimizethe degree of infection with some nematodes. Pasture contamination is reduced to an acceptable level by grazing sensitive animals with stock that is genetically or immunologically immune to helminths. Adult
cattle (immune) can thus graze alongside calves (susceptible). The grazing of resistant and susceptible
animals of the same species at separate times lowers pasture pollution. Trichostrongyle infections in calves (sensitive animals) can be decreased by transferring them to meadows that have been grazed bycows when clean grazing is not accessible in late summer and autumn (resistant).
Control of Biological Vectors: By removing the vectors, infectious diseases spread by biological vectors
can be controlled. Insecticides can be used to kill insect vectors. In addition, the vectors’ habitat can be
removed, for example, by drainage of the land to remove intermediate hosts of Fasciola hepatica, such as
snails. (Mantovani, 1992)
Control of Mechanical Vectors: Destruction and disinfection can control living organisms that
mechanically spread infectious agents. Insecticides, for example, can kill biting fleas that spread bacteria.
Biosecurity: The application of management practices that decrease the chances of infectious pathogens
gaining access to, or spreading within, a food animal production unit is referred to as “biosecurity.”
Biosecurity can also be applied at the national level to keep viruses and pests out of a country. Biosecurity
is especially important during epidemics to prevent infection from spreading from diseased farms to healthy animals on ‘clean’ farms.
Niche Filling: The existence of one organism in a niche can prohibit another creature from occupying it.
This is known as epidemiological interference. It has been studied in the chicken industry, where day-old
chicks were fed suspensions of endogenous intestinal microorganisms to prevent virulent Salmonella spp.,
Campylobacter jejuni, and Escherichia coli from colonizing their digestive tract. This management strategy does not foster antibiotic resistance, unlike preventive antibiotic chemotherapy. It has been effective in limiting Salmonella spp. colonization throughout Sweden but has had mixed results elsewhere. (Dasti etal., 2010)
Improvement in Environment, Husbandry and Feeding: Diseases of intensively farmed animals, notably cattle and pigs, are major current issues that can only be treated when epidemiological studies have discovered the variables linked to poor management. This argument is highlighted by the relatively high average levels of mastitis in dairy herds housed in straw-yards throughout the winter compared to cubicles, even though a proportion of the herds in straw-yards had very low mastitis. This demonstrates that it is not the housing system per se that is to be blamed, but rather disparities in how it is governed (the inadequate provision of damp bedding in this example).
Genetic Improvement: Many agricultural and companion animal illnesses have a changeable heritable
component. In the case of zoonoses, this is not the case. Alternatively, as in canine hip dysplasia, the condition may be determined by numerous factors, of which only one is inherited, such as growth rate, body type, and pelvic muscle mass. Early discovery and voluntary agreement by owners of sick animals
not to reproduce from the animals helps limit the frequency of such diseases. Radiography, for example,
is a well-established technique for detecting hip dysplasia.
Minimal Disease Methods: In intensively farmed livestock, the disease can be reduced by sanitizing
contaminated areas and treating infected animals or moving them from the unit. Non-infected animals
can be created by caesarian sections and the hatching of uninfected poultry eggs. The term “minimum
disease methods” refers to the combination of procedures. They’ve only been used commercially in pig
and poultry operations.
Reservoir Neutralization (Tesshome and Shimeles Abegaz Addis, 2019)
Isolation: Separation of people or animals that are infected from the others in such places and under such conditions for the duration of their communicability in order to prevent or limit direct and indirect transmissions of the infectious agent, from those who are infected to those who are vulnerable or those who may spread the agent to others.
Test and Slaughter: Deliberate slaughter of a small number of infected animals in the presence of a
large number of non-infected animals.
Depopulation or ‘Stamping Out Policy’: If the disease-causing agent is very contagious and/or rapidly
spreading among animals in the population, deliberate slaughter of all animals in the community from a designated geographical area without undertaking laboratory diagnosis.
Mass Therapy: To maintain herd immunity or an immunological belt, at least 80% of the population must be immunized. This can be accomplished by implementing a “ring vaccination campaign”
Conclusion
The Protection of animal, human and environmental health with the introduction of one health concept is believed to be future on achieving the healthy society. Improving the global capacity to respond for zoonotic disease and improving the early warning and surveillance systems using innovative technologies will lead to the control of emerging and transboundary migration of diseases. Information sharing between veterinary, health, wildlife and other aligned departments is required for effective surveillance to detect the onset of zoonotic illnesses. The expense and procedures for controlling the zoonotic disease are reduced if the condition is detected early. For the surveillance programme to be effective, all sectors should be involved in the planning process.To conclude One Health is a unified approach which will aid in devising appropriate control strategies to combat the emergence of new Zoonotic diseases.
References:
Arámbulo III P.V., Thakur A.S.1992. Impact of zoonoses in tropical America. Ann. N. Y. Acad.Sci. ;
653:6–18.
Atlas, R.M. One Health: Its origins and future. 2013. Curr. Top. Microbiol. Immunol. 2013, 365, 1–13.
[CrossRef] [PubMed]
Boqvist, S.; Söderqvist, K.; Vågsholm, I. Food safety challenges and One Health. within Europe. Acta
Vet.Scand. 2018, 60, 1. [CrossRef] [PubMed]
Cascio A., Bosilkovski M., Rodriguez-Morales A.J., Pappas G.2011. The socio-ecology of zoonotic infections. Clin. Microbiol. Infect. ;17:336–342.
Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U. 2010. Campylobacter jejuni: a brief overview on
pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. Apr;300(4):205-11
Garcia, S.N.; Osburn, B.I.; Cullor, J.S.2019. A one health perspective on dairy production and dairy food
safety.One Health , 7, 100086. [CrossRef] [PubMed]
Glaser CA, Angulo FJ, Rooney JA. 1994. Animal associated opportunistic infections among persons
infected with the human immunodeficiency virus. Clin Infect Dis. 18:14–2410.1093
Gregar, M., 2007 The human/ animal interface: emergence and resurgence of zoonotic infectious diseases.
Crit. Rev. Microbiol. 33: 243–299
Haregua Teshome and Shimeles Abegaz Addis, 2019. Review on Principles of Zoonoses Prevention,
Control and Eradication. Am J Biomed Sci & Res. 3(2)
Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. 2017. Antimicrobial drug
use in food-producing animals and associated human health risks: What, and how strong, is the
evidence? BMC Vet. Res. 13, 211. [CrossRef] [PubMed]
Keusch, G. T., Pappaioanou, M., Gonzalez, M. C., & National Research Council. 2009. Committee on
Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin. Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases,
Kumar R, Singh SP, Savalia CV. 2015.Overview of emerging zoonosis in India: Areas of concern. J Trop
Dis. 3:165‐8
Mackenzie, J.S.; McKinnon, M.; Jeggo, M. 2014. One Health: From Concept to Practice. In Confronting
Emerging Zoonoses: The One Health Paradigm; Yamada, A., Kahn, L.H., Kaplan, B., Monath, T.P., Woodall, J., Conti, L.,Eds.; Springer: Tokyo, Japan, :189. [CrossRef]
Mantovani A. Zoonoses control and veterinary public health. Rev Sci Tech. 1992 Mar;11(1):205-18
Marano, N., Arguin, P. M., & Pappaioanou, M. 2007. Impact of Globalization and Animal Trade on
Infectious Disease Ecology. Emerging Infectious Diseases, 13(12), 1807-1809.
Meslin F.X. . 2006 . Impact of zoonoses on human health. Vet. Ital; 42:369–379
Naveenkumar, V. , N ag, B . S. P. ,Vijayaraghavan, R. and Porteen, K. 2020. The Possible Risk of
Reverse Zoonos is in COVID-19: An Epidemiological Driving Approach for the One Health FutureChallenges: A Review. AsianJournal ofDairy and Food Research. 39(3): 173-179
Rabinowitz, P.M.; Natterson-Horowitz, B.J.; Kahn, L.H.; Kock, R.; Pappaioanou, M. 2017.Incorporating
One health into medical education. BMC Med. Educ. 17, 45. [CrossRef] [PubMed] © 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Singh B.B, Sharma R, Gill J.P.S, Aulakh R.S, Banga H.S. 2011. Climate change, zoonoses and India. Rev.
Sci.Tech. Off. Int. Epiz. 30:779–788
Thompson R.A.2013. Parasite zoonoses and wildlife: One health, spillover and human activity. Int.
J.Parasitol. 43:1079–1088
Welburn, S.C.; Beange, I.; Ducrotoy, M.J.; Okello, A.L. 2015. The neglected zoonoses–the case for
integrated control and advocacy. Clin. Microbiol. Infect. 21: 433–443. [CrossRef] [PubMed]