Pneumonic Pasteurellosis in Goats: An Overview
Anil Kumar Mishra1*, K Gururaj1, Nitika Sharma1, Anjali Singh1 and Vinay Chaturvedi1
1Animal Health Division, ICAR-Central Institute for Research on Goats, Makhdoom, Farah-281122, Mathura, Uttar Pradesh (India)
Introduction
Pneumonia is one of the most common respiratory problems in small ruminants throughout the world (Maria, 2007; Abera & Mossie, 2023), which is considered one of the most important causes of losses to goat farmers (Ferdausi et al., 2008; Mahdi et al., 2015). Pneumonia occurs when infectious and non-infectious agents (allergens, aspirations, etc.) cause the lungs of goats and sheep to become inflamed (Maria, 2007; Smith and Sherman, 2009). It is responsible for a very high morbidity and mortality in lambs and kids, especially in those that have not received adequate colostrum or in which passive colostral immunity is waning. It affects goats and sheep of all ages and causes severe morbidity manifested physically as unthriftiness or weight loss (Jarikre, 2013) and large-scale mortality in domestic and wild goats (Emikpe, 2013). Viruses, bacteria, fungi, parasites, mycoplasma, rickettsia, and chlamydia may cause pneumonia in goats (Smith and Sherman, 2009; Constable et al., 2017). Out of them, bacterial pneumonia is the most common and important in the case of animals, including small ruminants (Megra et al., 2006; Mekibib et al., 2019). Respiratory Syncytial virus, Parainfluenza virus type-3, PPR virus, Goat Pox virus, Retroviruses (Lentiviruses), Adenovirus, and Caprine Arthritis Encephalitis virus are the main viral agents associated with pneumonia in goats (Smith and Sherman, 2009; Constable et al., 2017). Cryptococcus spp. and Aspergillus spp. are the main fungal agents that are capable to cause pneumonia in sheep and goats (Carmo et al., 2020). Lungworms are the main parasitic agents associated with pneumonia (verminous pneumonia) in goats (Chakraborty et al., 2014). Mycoplasma capricolum subsp. Capripneumoniae is the main mycoplasmal agent responsible for causing pneumonia in goats (Ahaduzzaman, 2021). Bacterial pneumonia is caused by numerous bacterial pathogens such as Mannheimia haemolytica, Pasteurella multocida, Corynebacterium pseudotuberculosis, Trueperella pyogenes, Bibersteinia trehalosi, E. coli, Staphylococcus spp., Streptococcus spp., Bacillus spp., Klebsiella pneumoniae, Micrococcus spp., Rhodococcus spp., Acinetobacter spp., Citrobacter, Enterobacter spp., Hafnia spp., Proteus spp., Bordetella parapertussis, etc. (Sharma et al., 1991; Yimer and Asseged, 2007; Azizollah et al., 2009; Momin et al., 2011; Hanan and Hassan, 2012; Tijjani et al., 2012; Barde et al., 2016). Under bacterial pneumonia, pneumonic pasteurellosis is one of the most economically important infectious diseases of ruminants, with a wide prevalence throughout the continents (Maria, 2007; Abdullah, 2014; Legesse et al., 2018). Pneumonic pasteurellosis is caused by Mannheimia haemolytica, Pasteurella multocida, and Bibersteinia trehalosi (Maria, 2007; Smith and Sherman, 2009; Abdullah, 2014; Abera & Mossie, 2023).
Etiology
Pasteurella was originally named Bipolare multocidum in 1885. Pasteurella multocida, Mannheimia haemolytica, and Bibersteinia trehalosi are aerobic, gram-negative, tiny, ovoid rods, non-spore-forming coccobacilli of the family Pasteurellaceae. The bacteria inhabit the mucosal surfaces of the respiratory (mainly), gastro-intestinal and genital tracts of mammals. Only P. haemolytica causes hemolysis (β-hemolytic). Colonies are 1 to 2 mm in diameter. Pasteurella multocida, but not M. haemolytica, produces indole. Mannheimia (Pasteurella) haemolytica was previously divided into two biovars: biovar A, which ferments arabinose, and bivoar T, which ferments trehalose (Bingham et al. 1990). M. haemolytica includes 12 serotypes (biotypes), 1, 2, 5-9, 12-14, and 16-17, whereas P. multocida is classified into 5 serogroups (A, B, D, E, and F) based on capsular polysaccharides and 16 somatic types (1–16) based on LPS. A serotype is identified by its serogroup, followed by its somatic type, such as B:2 in the case of Pasteurella multocida. All of the A biotypes, with the exception of A11, were renamed Mannheimia haemolytica. A11 was given the name Mannheimia glucosida. M. haemolytica serotype A1 is the most commonly isolated bacteria in acute fibrinous pleuropneumonia associated with shipping fever (prevalence 65%–75%). P. multocida A:3 is the second most common bacteria isolated from acute to subacute fibrinopurulent bronchopneumonia in cattle (prevalence 21%–34%) and the major cause of bronchopneumonia in dairy calves (prevalence > 65). Subsequently, the T biovar was designated as Pasteurella trehalosi, and then the organism was assigned to a new genus, becoming Bibersteinia trehalosi (Blackall et al. 2007) in honor of Ernst Biberstein, who did much of the characterization work on the organism. The studies show that only leukotoxin-producing strains of B. trehalosi can cause pneumonia, and only about 15% of the B. trehalosi strains produce leukotoxin. Research by the authors shows that B. trehalosi replicates at almost twice the rate of M. haemolytica and is able to inhibit and overgrow M. haemolytica. This data would lead to speculation that, in many cases, M. haemolytica might be the initial cause of pneumonia but is then overgrown by B. trehalosi, which is the organism subsequently isolated (Dassanayake, 2010). M. haemolytica type A2 has been reported most frequently from pneumonic goats and sheep (Fodor et al., 1984; Midwinter et al., 1986). The T3, T4, T10, and T15 serotypes of B. trehalosi have been most often associated with the systemic or septicemic form of pasteurellosis affecting goats and sheep. These serotypes have been regrouped into B. trehalosi biotype 2, and a new biotype 4 has been added. B. trehalosi is often isolated from the lungs of sheep, goats, and cattle, but pathogenicity is variable and may be incidental. However, few reports say that it can cause pneumonia (Ngatia et al., 1986; Ward et al., 2002; Shiferaw et al., 2006). M. haemolytica is the most commonly isolated bacteria in pneumonic cases (Smith and Sherman, 2009). tracts of mammals. Only P. haemolytica causes hemolysis (β-hemolytic). Colonies are 1 to 2 mm in diameter. Pasteurella multocida, but not M. haemolytica, produces indole. Mannheimia (Pasteurella) haemolytica was previously divided into two biovars: biovar A, which ferments arabinose, and bivoar T, which ferments trehalose (Bingham et al. 1990). M. haemolytica includes 12 serotypes (biotypes), 1, 2, 5-9, 12-14, and 16-17, whereas P. multocida is classified into 5 serogroups (A, B, D, E, and F) based on capsular polysaccharides and 16 somatic types (1–16) based on LPS. A serotype is identified by its serogroup, followed by its somatic type, such as B:2 in the case of Pasteurella multocida. All of the A biotypes, with the exception of A11, were renamed Mannheimia haemolytica. A11 was given the name Mannheimia glucosida. M. haemolytica serotype A1 is the most commonly isolated bacteria in acute fibrinous pleuropneumonia associated with shipping fever (prevalence 65%–75%). P. multocida A:3 is the second most common bacteria isolated from acute to subacute fibrinopurulent bronchopneumonia in cattle (prevalence 21%–34%) and the major cause of bronchopneumonia in dairy calves (prevalence > 65). Subsequently, the T biovar was designated as Pasteurella trehalosi, and then the organism was assigned to a new genus, becoming Bibersteinia trehalosi (Blackall et al. 2007) in honor of Ernst Biberstein, who did much of the characterization work on the organism. The studies show that only leukotoxin-producing strains of B. trehalosi can cause pneumonia, and only about 15% of the B. trehalosi strains produce leukotoxin. Research by the authors shows that B. trehalosi replicates at almost twice the rate of M. haemolytica and is able to inhibit and overgrow M. haemolytica. This data would lead to speculation that, in many cases, M. haemolytica might be the initial cause of pneumonia but is then overgrown by B. trehalosi, which is the organism subsequently isolated (Dassanayake, 2010). M. haemolytica type A2 has been reported most frequently from pneumonic goats and sheep (Fodor et al., 1984; Midwinter et al., 1986). The T3, T4, T10, and T15 serotypes of B. trehalosi have been most often associated with the systemic or septicemic form of pasteurellosis affecting goats and sheep. These serotypes have been regrouped into B. trehalosi biotype 2, and a new biotype 4 has been added. B. trehalosi is often isolated from the lungs of sheep, goats, and cattle, but pathogenicity is variable and may be incidental. However, few reports say that it can cause pneumonia (Ngatia et al., 1986; Ward et al., 2002; Shiferaw et al., 2006). M. haemolytica is the most commonly isolated bacteria in pneumonic cases (Smith and Sherman, 2009).
Pathogenesis
M.hemolytica, B. trehalosi,and P. multocida aredistributed worldwide, and diseases caused by them are common in sheep and goats of all ages, although the prevalence of serotypes may vary by region and flock. The presence of multiple Pasteurella species may serve to keep the bacterial populations in check because there appears to be some interference with growth when multiple species are present. For these organisms to cause infection, a combination of stressors such as poor ventilation (mainly), heat, steroid administration, overcrowding, parasites (worms and coccidia), kidding, high humidity, dusty damp bedding, illness, fright, pregnancy, weaning, poor housing conditions, aspiration of liquids, irritant dust and fumes, change of diet, inadequate nutrition, commingling with animals from unrelated farms, exposure to inclement weather, lung parasitism, pulmonary adenocarcinoma, handling, transportation, etc. play a very vital role (Mugera and Kramer, 1967; Jasni et al., 1991; Brogden et al., 1998; Maria, 2007). The combination of stressors and primary infections such as viral infections (Parainfluenza 3, adenovirus type 6, respiratory syncytial virus, bovine adenovirus type 2, ovine adenovirus types 1 and 5, reovirus type 1), bacterial infections (Mycoplasma ovipneumoniae, caseous lymphadenitis, and Bordetella parapertussis), and fungal infections are thought to break down the mucosal barrier integrity of the lower respiratory tract, allowing M. haemolytica, B. trehalosi, and P. multocida to colonize, proliferate, and induce significant tissue damage. Further, the virulence of M. haemolytica, P. multocida, and B. trehalosi is mediated by the action of several factors, including endotoxin, leukotoxin, and capsular polysaccharide. Pathogen-host interactions result in tissue damage, especially because of the massive influx of neutrophils. Enzymes released by the dying neutrophils cause additional injury to lung tissue. Leukotoxin is particularly important in pathogenesis because it is specifically toxic to ruminant leukocytes (mainly neutrophils and macrophages), resulting in fibrin deposition in the lungs and on pleural surfaces. The lipopolysaccharide endotoxin contributes to adverse reactions in the lungs and leads to systemic circulatory failure and shock. The capsular polysaccharide prevents the phagocytosis of the bacteria and assists in attachment to the alveolar epithelial surface. Survival in the acute phase of pneumonic pasteurellosis depends on the extent of lung involvement and damage to the lower respiratory tract. Sheep and goats that recover may have chronic respiratory problems, including reduced lung capacity and weight gain efficiency, if ≥ 20% of the lung is damaged. Pasteurellosis during an outbreak may spread to unstressed herd members (Pande, 1943; Buddle et al., 1990).
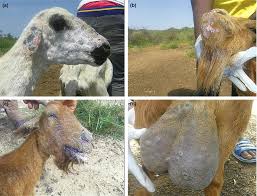
Clinical Findings and Lesions
B.trehalosimainly causes septicemia and systemic pasteurellosis in sheep below 2 months old. The systemic form of pasteurellosis is characterized by fever (104°F to 106°F), mucopurulent nasal and ocular discharge, lethargy, anorexia, dyspnea, and a moist, painful cough. Auscultation may reveal crackles, areas of consolidation (increased bronchial tones), or pleuritis (friction rub early, muffled sounds later). Mortality rates may be 10% or more. Commonly, goat is found suddenly dead before showing any signs. The organism is thought to move from the tonsils to the lungs and pass into the blood. This results in septicemia and localization of the infection in one or more tissues, such as the joints, udder, meninges, or lungs. P. multocida hasbeen reported to be isolated from polyarthritis in young lambs. M. haemolytica has been reported in cases of mastitis, especially in sheep. All of these bacteria can cause severe fibrinonecrotic pneumonia in sheep and goats. The disease is characterized by an acute onset of illness, very high fevers, dyspnea, anorexia, and often death. Rumen contractions are reduced or absent. There may be evidence of diarrhea and dehydration. Frothy fluid may be noted around the mouth during the terminal stages.
Diagnosis
At necropsy, testing whether lung tissue sinks (pneumonia) or floats (normal) remains a useful screening test. PM Lesions include subcutaneous hemorrhage; epithelial necrosis of the tongue, pharynx, esophagus, or occasionally the abomasum and intestine; enlargement of tonsils and retropharyngeal lymph nodes; and per acute, multifocal, embolic, necrotizing lesions in the lung and liver. There is a red to purple consolidation of the lung lobes, sometimes accompanied by a fibrinous pleuritis. Histologic changes are typical of pasteurellosis in other species and include hemorrhage, necrosis, and exudation of fibrin, edema fluid, and neutrophils or macrophages into the airways.
Treatment
Commonly recommended antibiotics include oxytetracycline (10 mg/kg/day of the non-long-acting product or 20 mg/kg once of the long-acting product), penicillin (20,000 to 40,000 IU/kg once daily), ampicillin (5 to 10 mg/kg twice daily), tetracycline (5 mg/kg once or twice daily), tylosin (10 to 20 mg/kg once or twice daily), ceftiofur (1.1 to 2.2 mg/kg daily), and florfenicol (40 mg/kg every one to two days SC) for 3–5 days. tilmicosin, danofloxacin, enrofloxacin, trimethoprim-sulfamethoxazole, and tulathromycin may also be used. Treatment is frequently unrewarding unless begun very early in the disease process because of the rapid progression of lung damage and endotoxin release. Parenteral fluids and anti-inflammatory agents (flunixin meglumine or ketoprofen) are important adjuncts to antibiotic therapy.
Prevention
No bacterin has been proven effective against pasteurellosis in goats. No vaccine against Mannheimia hemolytica or Biebersteinia trehalosi is available in India. A vaccine against Pasteurella multocida is available in India, contains an inactivated culture of Pasteurella multocida, is given at a rate of 2 ml subcutaneously, and is repeated semi-annually. For the prevention of pneumonic pasteurellosis, attention must be given to proper ventilation, housing, and nutrition. Proper colostrum intake must be ensured. Dampness in the housing must be removed. In the winter season, the new-born kids should be protected from the cold without interfering with the ventilation of the housing. Crowding must be avoided. Strict isolation of purchased animals or those stressed by traveling is recommended. In general, exposure to the stressors mentioned at the start of the article must be avoided.
References
- Abdullah FF, Tijjani A, Adamu L, Teik Chung E, Abba Y, Mohammed K, Saharee A, Haron A, Sadiq MA and Mohd AM (2014). Pneumonic pasteurellosis in goat. Iranian Journal of Veterinary Medicine, 8 (4):293-296.
- Abera, D., & Mossie, T. (2023). A review on pneumonic pasteurellosis in small ruminants. Journal of Applied Animal Research, 51(1), 1-10.
- Ahaduzzaman, M. D. (2021). Contagious caprine pleuropneumonia (CCPP): A systematic review and meta‐analysis of the prevalence in sheep and goats. Transboundary and Emerging Diseases, 68(3), 1332-1344.
- Azizollah E, Bentol-hoda M and Razieh K (2009). The aerobic bacterial population of the respiratory passageways of healthy dromedaries in Najaf-abbad abattoir, Central Iran. Journal of Camelid Science, 2: 26-29.
- Barde P, Garg UK, Sharda R, Chhabra D and Shukla S (2016). Isolation and identification of bacterial pathogens from respiratory tract of goats. The Indian Journal of Veterinary Sciences & Biotechnology, 12 (3):138-140.
- Bingham DP, Moore R and Richards AB (1990). Comparison of DNA: DNA homology and enzymatic activity between Pasteurella haemolyticaand related species. American Journal of Veterinary Research, 51 (8): 1161-1166.
- Blackall PJ, Bojesen AM, Christensen H and Bisgaard M (2007). Reclassification of [Pasteurella] trehalosias Bibersteinia trehalosi nov., comb. nov. International Journal of Systematic and Evolutionary Microbiology, 57 (4): 666-74.
- Brogden KA, Lehmkuhl HD and Cutlip RC (1998): Pasteurella haemolytica complicated respiratory infections in sheep and goats. Veterinary Research, 29: 233–254.
- Buddle BM, Pfeffer A, Cole DJ, Pulford HD and Ralston MJ (1990). Experimental respiratory infection of goats with caprine Herpesvirus and Pasteurella haemolytica. New Zealand Veterinary Journal, 38 (1): 22-27.
- Carmo PMSD, Uzal FA, Pedroso PMO, Riet-Correa F. Conidiobolomycosis, cryptococcosis, and aspergillosis in sheep and goats: a review. J Vet Diagn Invest. 2020 Nov; 32(6):826-834. doi: 10.1177/1040638720958338. Epub 2020 Sep 14. PMID: 32921278; PMCID: PMC7649532.
- Chakraborty S, Kumar A, Tiwari R, Rahal A, Malik Y, Dhama K, Pal A and Prasad M (2014). Advances in diagnosis of respiratory diseases of small ruminants. Veterinary Medicine International(doi:10.1155/2014/508304).
- Constable P. D., Hinchcliff K.W. Stanley H.D. Grünberg W. (2017). Veterinary Medicine, a textbook of disease of cattle, sheep, goats, pigs and horses. The English Language Book Society and Bailliere Tindall. 11th
- Dassanayake RP, Call DR, Sawant AA, Casavant NC, Weiser GC, Knowles DP and Srikumaran S (2010). Bibersteinia trehalosiinhibits the growth of Mannheimia haemolytica by a proximity-dependent mechanism. Applied and Environmental Microbiology, 76 (4): 1008-13.
- Emikpe BO, Jarikre TA and Eyarefe OD (2013). Retrospective study of disease incidence and type of pneumonia in Nigerian small ruminants in Ibadan, Nigeria. African Journal of Biomedical Research, 16 (2): 107-113.
- Ferdausi T, Haider MG, Alam KJ, Baki MA and Hossain MM (2008). Caprine lung diseases and causal bacteria. The Bangladesh Veterinarian25 (1): 9–16.
- Fodor L, Varga J, Hajtos I and Szemeredi G (1984). Serotypes of Pasteurella haemolyticaisolated from sheep, goats and calves. Zentralblatt für Veterinärmedizin Reihe B, 31: 466-469.
- Hanan ME and Hassan SO (2012). Pneumonia in Goats in Sudan. International Journal of Animal and Veterinary Advances,4 (2):144-145.
- Jarikre TA, Akpavie SO and Bello K (2013). Outbreak of contagious bovine pleuropneumonia in Ibarapa, South West Nigeria. Tropical Veterinarian, 31 (1): 20-29.
- Jasni S, Zamri-Saad M, Mutalib AR & Sheikh-Omar AR (1991). Isolation of Pasteurella haemolytica from the nasal cavity of goats. British Veterinary Journal,147 (4): 352-355.
- Mahdi AA, Al-Naqshabendy AA and Haddel BT (2015). A study of some pathological lesions in the lung of sheep and Duhok abattoir. Basrah Journal of Veterinary Research, 14 (2):265-277.
- Maria L (2007). Bacterial pneumonia in goats. In: Alabama Cooperative Extension System online publication. www.aces.edu/urban.accessed 26/1/ 2014.
- Maria L (2007). Bacterial pneumonia in goats. In: Alabama Cooperative Extension System online publication. www.aces.edu/urban.accessed 26/1/ 2014.
- Megra T, Sisay T and Asseged B (2006). The aerobic bacterial flora of the respiratory passageways of healthy goats in Dire Dawa Abattoir, Eastern Ethiopia. Revue de Medecine Veterinaire, 157 (2): 84-87.
- Mekibib B, Mikir T, Fekadu A, Abebe R. 2019. Prevalence of pneumonia in sheep and goats slaughtered at Elfora Bishoftu Export Abattoir, Ethiopia: a pathological investigation. J Vet Med. 1–10. https://doi.org/ 10.1155/2019/5169040.
- Midwinter AC, Clarke JK and Alley MR (1986). Pasteurella haemolyticaserotypes from pneumonic goat lungs. New Zealand Veterinary Journal, 34: 35–36.
- Momin MA, Islam MA, Khatun MM, Rahman MM and Islam MA (2011). Characterization of bacteria associated with pneumonia in black Bengal goats. Bangladesh Journal of Veterinary Medicine, 9 (1): 67–71.
- Mugera, GM & Kramer TT (1967). Pasteurellosis in Kenya goats due to Pasteurella haemolytica. Bulletin des epizooties en Afrique, 15 (2): 125-131.
- Ngatia TA, Kimberling CV, Johnson LW, Whiteman CE and Lauermann Jr LH (1986). Pneumonia in goats following administration of live and heat-killed Pasteurella haemolytica. Journal of Comparative Pathology, 96 (5): 557-64.
- Pande PG (1941). Pleuropneumonia in goats with special reference to Pasteurella Indian Journal of Veterinary Science andAnimal Husbandry, 13: 44-58.
- Sharma RK, Boro BR and Borah P (1991). Incidence of caprine pneumonia associated with bacteria. Indian journal of animal sciences 61(1):54-55.
- Shiferaw G, Tariku S, Ayelet G and Abebe Z (2006). Contagious caprine pleuropneumonia and Mannheimia haemolytica-associated acute respiratory disease of goats and sheep in Afar Region, Ethiopia. Revue Scientifique et Technique-Office International des Epizooties, 25 (3): 1153-1163.
- Smith MC and Sherman DM (2009).Respiratory System: In Goat Medicine. Wiley-Blackwell ublication, Iowa, 2ndedition, pp. 354-355.
- Tijjani AN, Ameh JA, Gambo HI, Hassan SU, Sadiq MA and Gulani I (2012). Studies on the bacterial flora and pathologic lesions of caprine pneumonic lungs in Maiduguri North-Eastern Nigeria. African Journal of Microbiology Research, 6 (48): 7417-7422.
- Ward AC, Weiser GC, DeLong WJ and Frank GH (2002). Characterization of Pasteurella isolated from healthy domestic pack goats and evaluation of the effects of a commercial
- Yimer N and Asseged B (2007). Aerobic bacterial flora of the respiratory tract of healthy sheep slaughtered in Dessie municipal abattoir, North-Eastern Ethiopia. Revue de Medecine Veterinaire, 158 (10): 473-478.