Presence of Antibiotic Residues in livestock product and public Health Hazard
Dr.Kedar Karki,VS,Nepal
Background:
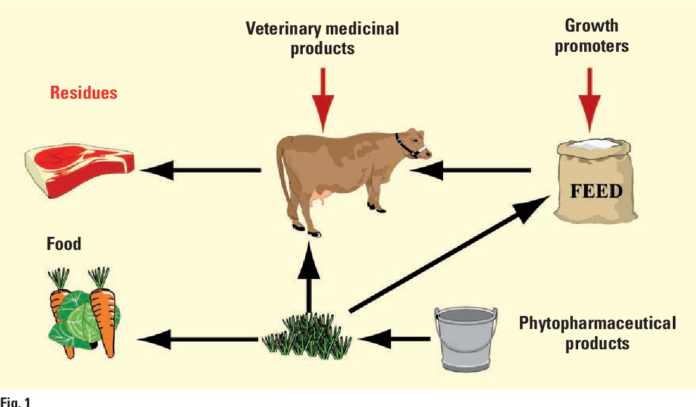
Use of Antibiotic that might result in deposition of residues in meat, milk and eggs must not be permitted in food intended for human consumption. If use of antibiotics is necessary as in prevention and treatment of animal diseases, a withholding period must be observed until the residues are negligible or no longer detected. The use of antibiotics to bring about improved performance in growth and feed efficiency, to synchronize or control of reproductive cycle and breeding performance also often lead to harmful residual effects. Concern over antibiotic residues in food of animal origin occurs in two times; one which produces potential threat to direct toxicity in human, second is whether the low levels of antibiotic exposure would result in alteration of microflora, cause disease and the possible development of resistant strains which cause failure of antibiotic therapy in clinical situations. A withdrawal period is established to safeguard human from exposure of antibiotic added food. The withdrawal time is the time required for the residue of toxicological concern to reach safe concentration as defined by tolerance. It is the interval from the time an animal is removed from medication until permitted time of slaughter. Heavy responsibility is placed on the veterinarian and livestock producer to observe the period for a withdrawal of a drug prior to slaughter to assure that illegal concentration of drug residue in meat, milk and egg do not occur. Use of food additives may improve feed efficiency 17% in beef cattle, 10% in lambs, 15% in poultry and 15% in swine. But their indiscriminate use will produce toxicity in consumers. WHO and FAO establish tolerances for a drug, pesticide or other chemical in the relevant tissues of food producing animals. The tolerance is the tissue concentration below, which a marker residue for the drug or chemical must fall in the target tissue before that animal edible tissues are considered
Safe for human consumption. Tolerances are established based on extensive toxicological studies of potential hazards of consumption to human.
Antibiotics as Growth Promoter.
The antibiotics nowadays used for improved performance in growth especially in broilers and fatteners. They may produce improved growth rate because of thinning of mucous membrane of the gut, facilitating better absorption, altering gut motility to enhance better assimilation, producing favorable conditions to beneficial microbes in the gut of animal by destroying harmful bacteria and partitioning proteins to muscle accretion by suppressing monokines. Antibiotics also favor growth by decreasing degree of activity of the immune system, reduced waste of nutrients and reduce toxin formation. In most of the cases only young growing animals and poultry are responsive to antibiotic mediated growth promotion.
Antibiotics in Therapeutics:
Indiscriminating use of antibiotics in all cases of pyrexia, inflammation, wounds and viral diseases have widespread residual effects on edible tissues. The use of antibiotics only in specific conditions is justified because the roll of microbial agents is mainly to kill the rapidly dividing invading cells.
Antibiotics in Prophylaxis:
Animals and poultry are receiving subtherapeutic levels of antibiotics to prevent possible infection. But the antibiotics are specific to their spectrum of activity only in the active multiplying stage of bacteria. But it will not provide overall protection. Only in certain cases like dry cow therapy and surgical procedures are wanting of antibiotic prophylaxis.
Miscellaneous use of Antibiotics:
Antimicrobials are used either directly or indirectly during the production processing and storage of milk and milk products. Direct contamination of milk may occur from air and water during processing, storage and transportation. Besides feed given to animals is also source of indirect contamination. Man will be the ultimate consumer of these antibiotic residues. There are some causes of miscellaneous use like lack of awareness, lack of extension activities, inadequate literature supplied by manufacturers, lack of safer drugs and exploitation of more production and profit from animals. The extra label use of chloramphenicol, furazolidone, nitrofurazone, sulphonamide drugs, and flouroquinolones in lactating animals is prohibited.
Pathological Effects produced by Antibiotic Residues in Food:
– Transfer of antibiotic resistant bacteria to the human.
– Immunopathological effects
– Autoimmunity
– Carcinogenicity (Sulphamethazine,
Oxytetracycline, Furazolidone)
– Mutagenicity
– Nephropathy (Gentamicin)
– Hepatotoxicity
– Reproductive disorders
– Bone marrow toxicity (Chloramphenicol)
– Allergy (Penicillin)
Residues Prevention:
The first step in residue prevention is to make individuals and organizations aware of the problem through education by veterinary personnel, organizations, and literatures and governmental agencies. Rapid screening procedures for the analysis of antibiotic residues and instant grading and prohibition of food containing antibiotics more than minimum residue level. Processing of milk help for the inactivation of antibiotics. Refrigeration causes disappearance of penicillin. In pasteurization most of antibiotics will lose activity. Use of activated charcoal, resin and UV irradiation also help for antibiotic inactivation. Irrational use of antibiotics in field veterinary practices should be avoided. Development of simple and economic field test to identify drug residue in edible animal products. Ethno-veterinary practices may be promoted. Nationwide monitoring and periodic surveillance of microbial residue in edible tissues and milk.
VETERINARY DRUG RESIDUE IN LIVESTOCK FOOD PRODUCTS & ITS RISK FACTORS ON PUBLIC HEALTH