PREVENTION AND CONTROL OF ZOONOSES TO ACHIEVE ONE WORLD – ONE HEALTH
- Nagendra Reddy1, G Shalini
Ph.D Scholar, Department of Animal Reproduction, Gynaecology and Obstetrics, CVAS, Mannuthy, Thrissur, Kerala
Corresponding author mail id: nagendrareddyy93@gmail.com
- INTRODUCTION:
“Zoonoses” is derived from the Greek word “Zoon” which means animal, and “nosos” means illness. Zoonosis is “any infection or disease that is transmissible naturally to humans from vertebrate animals” and that infection or disease is maintained in an animal (reservoir) population and serves as a continuous source of human infections (WHO,2020). Over 60% of diseases that affect humans have zoonotic origins (Taylor et al., 2001). This includes numerous types of bacteria, viruses, fungi, protozoa, parasites, and other pathogens (Morse et al., 2012). Three distinct typologies of interventions—animal-assisted activity (AAA), animal-assisted education (AAE), and animal-assisted therapy (AAT)—that involve experts with advancing levels of training and in which animals interact with people and patients more closely (ING, 2015). A possible epidemiological role for seemingly healthy animals implicated in AAIs could be in the asymptomatic carriage and possibly transmission of zoonotic infections to humans (Gerandi et al., 2018). This includes infections that human’s contract from direct contact with animals as well as infections that are spread indirectly through vector-borne, environmental, or food system pathogens. The emergence, reemergence, distribution, and patterns of zoonoses have been significantly influenced by a number of factors, including climate change, urbanisation, animal migration and trade, travel and tourism, vector biology, anthropogenic factors, and natural factors. The ongoing COVID-19 pandemic, which is “the most serious challenge of the post-war era due to the sudden halt in economic activity in both advanced and developing countries,” is the most striking and recent example of the global economic impact of a zoonosis. (UNIDO, 2020). A wide range of diseases with complex life cycles involving a variety of hosts and occasionally vectors or parasites are caused by zoonotic infections. These infections have a significant negative influence on both human and animal health locally and globally, as well as having a significant socioeconomic impact on endemic populations. This impact has recognised recently recognized and leads to global initiatives such as the ‘One Health’ approach, resulting in a thorough examination of the viewpoints of both humans and animals and robust cooperation between the environmental health sectors (CDC, 2020). In this article I would like to focus the etiological agents responsible for zoonoses and prevention and control of zoonotic parasites to achieve one world-one health concept.
- Classification of Zoonoses:
The older classification of zoonoses includes the terms anthropozoonoses, zooanthroponoses, amphixenoses, and euzoonoses (Hubalek, 2003). Anthropozoonoses are animal diseases that can be transmitted to humans, such as rabies. Zooanthropozoonoses defined as those diseases that are transmitted to animals from humans such as tuberculosis in cat and monkey. Amphizoonoses are those diseases that can be transmitted in any direction (from human to animal and from animal to human) such as staphylococcal infection. For some parasitic diseases, Euzoonoses are those parasitic diseases where humans act as the obligatory host such as taenia solium and taenia saginata infections.
Based on etiology, zoonoses are classified into bacterial zoonoses (anthrax, brucellosis etc.), viral zoonoses (Ebola, avian influenza etc.,), parasitic zoonoses (toxoplasmosis, giardiasis, malaria etc.,), fungal zoonoses (ring worm), rickettsial zoonoses (Q-fever), chlamydial zoonoses (psittacosis), mycoplasma zoonoses (Mycoplasma pneumoniae infection), protozoal zoonoses and diseases caused by acellular non-viral pathogenic agents (Transmissible spongiform encephalopathies and mad cow disease) (Chomel, 2009).
- Lists the major zoonotic diseases with their etiological agents, animal host, and major symptoms in humans (Rahman et al., 2020)
3.1 Bacterial zoonoses:
| Disease | Etiology | Animal Host | Major Symptoms, System or Organs Involved |
| Brucellosis | Brucella abortus Brucella melitensis, Brucella suis, Brucella canis, | Cattle, goats, sheep, pigs, and dogs | Fever usually high in the afternoon, back pain, joint pain, poor appetite, and weight loss |
| Anthrax | Bacillus anthracis | Cattle, horses, sheep, pigs, dogs, bison, elks, white-tailed deer, goats, and mink | Skin, respiratory organs or GI tract |
| Tuberculosis | Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti | Cattle, sheep, swine, deer, wild boars, camels, and bison | Respiratory organs bone marrow |
| Glanders | Burkholderia mallei | Horses, donkeys, and mules | Fever, sweating, muscle aches, chest pain, muscle tightness, and headache |
| Salmonellosis | Salmonella enterica, Salmonella bongor | Domestic animals, birds, and dogs | Enteritis |
| Pasteurellosis | Pasteurella multocida | Poultry, pigs, cattle, buffaloes, sheep, goats, deer, cats, dogs, and antelope | Fever, vomiting, diarrhea, and gangrene |
| Leptospirosis | Leptospira interrogans | Wild and domestic animals including pet dogs | Fever, abdominal pain, |
| Tularemia | Francisella tularensis | Rabbits, squirrels, muskrats, deer, sheep, bull snakes, wild rodents, beavers, cats, and dogs | Joint pain, diarrhea, and dry cough |
| Bubonic plague | Yersinia pestis | Rock squirrels, wood rats, ground squirrels, prairie dogs, mice, voles, chipmunks, and rabbits | Fever, chills, abdominal pain, diarrhea, vomiting, and bleeding from natural opening |
| Enterohemorrhagic Escherichia coli infections | E coli O157:H7 | Cattle, sheep, pigs, deer, dogs, and poultry | Enteritis and Haemolytic–uremic syndrome (HUS) |
3.2 Viral zoonoses:
| Rabies | Rabies virus | Cattle, horses, cats, dogs, bats, monkeys, wolves, skunks, rabbits | Nervous system |
| Newcastle disease | Paramyxovirus | Poultry and wild birds | Conjunctivitis |
| Avian influenza | Influenza A virus | Ducks, chickens, turkeys, dogs, cats, pigs, whales, horses, seals, and wild birds | Flu like symptoms, diarrhea, and pneumonia |
| Ebola virus disease (Ebola Hemorrhagic Fever) | Ebola virus | Monkeys, gorillas, chimpanzees, apes, and wild antelopes | Fever, intense weakness, muscle pain, headache, sore throat, hemorrhage, vomiting, diarrhea, kidney, and liver failure |
| Chikungunya fever | Chikungunya virus | Monkeys, birds, and rodents | High fever, severe joint pain, muscle pain, and skin rash |
| Dengue fever | Dengue virus | Monkeys and dogs | High fever, skin rash, skin hemorrhage, and shock |
| Severe acute respiratory syndrome (SARS) | SARS coronavirus (SARS-CoV) | Bats, dogs, cats, ferrets, minks, tigers, and lions | influenza-like symptoms, fever, muscle pain, severe cases progress to a respiratory disease and pneumonia |
| Rift Valley fever | Rift Valley fever virus | Buffaloes, camels, cattle, goats, and sheep | Influenza- like fever, muscle pain, joint pain, and headache |
3.3 Parasitic zoonoses:
| Fascioliasis | Fasciola hepatica, Fasciola gigantica | Cattle, sheep, goats, and other ruminants | Intense internal bleeding, fever, nausea, swollen liver, skin rashes, and extreme abdominal pain |
| Hydatidosis | Echinococcus granulosus | Buffaloes, sheep, goats and adult stray or shepherd dogs | Hydatid cysts in liver, lungs, bones, kidneys, spleen, abdominal pain, and respiratory problem |
| Cryptosporidiosis | Cryptosporidium parvum | Cattle, sheep, pigs, goats, horses, and deer | Diarrhea lasting 3–14 days. Abdominal pain, nausea and malaise are frequent. Some patients have a slight fever |
| Cutaneous larval migrans | Ancylostoma braziliense | Dogs and cats | Subcutaneous tissue |
| Visceral larva migrans | Toxocara canis, Toxocara cati, and Ascaris suum | Birds, emus, cats, dogs, rabbits | Gastrointestinal, e.g., coughing, shortness of breath, fever, and abdominal pain |
3.4 Acellular non-viral pathogenic agents:
| Mad Cow Disease, also known as BSE (Bovine spongiform encephalopathy). In human known as Creutzfeldt–Jakob disease (CJD) | Prion protein | Cattle, sheep, goats, mink, deer, and elks | Ataxia, jerky movements, seizures, dementia, memory loss, and personality changes |
3.5 Mycotic/fungal zoonoses:
| Tinea/ringworm infection | Microsporum spp., Trichophyton spp. | All animals like cattle, sheep, goats, cats, and dogs | Skin lesions |
| Aspergillosis | Aspergillus spp. | All domestic animals and birds | Respiratory problems |
| Malassezia infection | Malassezia spp. | Dogs and cats | Pityriasis versicolor, seborrheic dermatitis, atopic eczema, folliculitis, and dandruf |
| Histoplasmosis | Histoplasma capsulatum var. capsulatum | Cats, dogs, rabbits, and rats | Often asymptomatic, fever, productive cough, chest pain, weight loss, hepatosplenomegaly, and hematologic disturbances |
| Coccidioidomycosis | Coccidioides immitis, Coccidioides posadasii | Dogs, horses, pigs, and ruminant | Hypersensitivity reaction, fever, erythema nodosum, erythema multiform, arthralgia, pleuritic chest pain, and dry cough |
3.6 Protozoal zoonoses:
| Trypanosomiasis | Trypanosoma brucei | Antelopes, cattle, camels, and horses | chronic and intermittent fever, headache, pruritus, lymphadenopathy, hepatosplenomegaly, and sleep disturbance |
| Leishmaniasis | Leishmania infantum | Cats, dogs, horses, and bats | Skin lesions, hepatosplenomegaly, and wasting |
| African sleeping sickness | Trypanosoma brucei | Antelopes, cattle, camels, and horses | High fever, headache, nausea, vomiting, and erythematous plaque formation |
| Toxoplasmosis | Toxoplasma gondi | Pigs, sheep, goats, poultry, and rabbits | Lymphadenopathy, fever, malaise, night sweats, myalgia, sore throat, and maculopapular rash |
| Giardiasis | Giardia lamblia | Dogs, cats, ruminants, and pigs | Diarrhea, abdominal cramping, bloating, flatulence, malaise, nausea, and anorexia |
- Reverse Zoonoses:
Zoonotic diseases that are transmitted to humans from animals and then back from humans to animals are referred as reverse zoonoses (Olayemi et al., 2020)
Examples of reverse zoonoses:
| Agent | Human Disease | Animal Disease | Animal Affected |
| Infectious hepatitis | Hepatitis | Hepatitis | Nonhuman primates |
| Staphylococcus aureus | Furunculosis | Furunculosis, mastitis | Cattle |
| Streptococcus pyogenes | Pharyngitis, scarlet fever | Mastitis | Cattle |
| Giardia lamblia | Nausea, flatulence diarrhoea | None known | Beavers |
| Mycobacterium tuberculosis | Tuberculosis | Tuberculosis | Deer, dogs, elephants |
| Mumps virus | Mumps | Parotiditis | Dogs |
For prevention and control of above zoonotic diseases the concept of one health – one world needs to be implemented all across the globe.
- ONE HEALTH CONCEPT:
One Health (OH) is an integrated concept in addressing zoonotic diseases in improving the efficiency and effectiveness of zoonosis prevention and control at global health level. The One Health implementation takes into account ecological and social variables that contribute to the spread of zoonosis and offers complete solutions to evaluate and manage associated risks in various communities based on customs and locations (He et al., 2022).
Prevention is defined as inhibiting the entry of a disease agent into a specific population group or an individual. Efforts done to bring a disease problem down to a manageable level and keep it there are referred to as control. Eradication is the final step in a disease control program. It consists of the elimination of a disease producing agent from a defined population or geographical area. Prevention and control referred to as “primary prevention” and “secondary prevention”. Primary prevention is aimed at maintaining a healthy population i.e. preventing the occurrence of a disease. Secondary prevention attempts to minimize damage after disease has occurred. Rehabilitation referred to as “tertiary prevention” (Sohn et al., 2003).
The principles of zoonoses prevention control and eradication programs are focused mainly on breaking the transmission chain and the reservoir, transmission from the reservoir to the susceptible hosts, and the susceptible hosts are the factors involved (Martinma, 2007).
5.1 Reservoir Neutralization:
Source of zoonotic infection is the infected reservoir host and when the infection in the reservoir can be reduced or eradicated and the sources of infection progressively reduces and disappear.
Three methods used to neutralize the reservoir are removing infected individual, rendering infected individuals (non-shedder) and manipulating the environment.
- Removal of infected individual:
This can be achieved in two ways: test and slaughter and mass therapy (Martinma, 2007). Infection may be removed from an infected herd by testing and slaughtering. This method has been successfully used to control bovine brucellosis and tuberculosis as well as equine dourine and glanders. By sensitive and specific tests all infected animals need to be detected and erased without removing large numbers of false-positive animals. This method has been most effective with agents spread by direct transmission and with limited number of reservoir species are involved. Due to high cost this method has prevented its usage in many countries (Schellenberg et al., 2003).
The second method for neutralizing the reservoir is by mass therapy. Mass treatment is often limited to a local setting where all animals or humans who may be affected are treated without first undergoing tests to determine who is actually infected. One example of mass therapy is treating every dog in a certain area to prevent echinococcosis. (Acha and Szyfres, 2003). Mass treatment is reducing many of the issues related to human intestinal parasites. Diethylcarbarnazine is being used to control fascioloiasis (Ibrahim, 2010) and mass therapy has decreased the incidence of Schistosomiasis and African trypanosomiasis (McQuiston and Childs, 2002).
By decreasing the agent’s survivability in the vector of vehicle (food, water, soil, plants), environmental manipulation is a reservoir neutralisation technique intended to break the chain of transmission between the portal of exit of the diseased (shedder) host and the vulnerable host. Proper disposal of faeces (acting on the portal of exit), disinfection of faeces, and pasture rotation to reduce exposure of susceptible hosts (portal of entry) are a few parasite management measures that serve as examples of this strategy. (Martinma, 2007).
5.2 Reducing Contact Potential:
Reducing the likelihood of contact is a fundamental strategy for preventing the direct transfer of an infectious agent from an infected person to a vulnerable host. Two populations are taken into account in disease control: the known infected and the possibly exposed susceptible. Three strategies are employed: population control, quarantine of potentially infected individuals, and isolation and treatment of patients.
Isolation (Quarantine) of an infected ill animal has two advantages; it reduces the probability of contact with a susceptible lost and facilitates treatment and disinfection because because this strategy depends on early and accurate diagnosis from successful disease control programmes based on isolating and treating cases to prevent the spread of infectious disease agents, it has serious limitations and frequently fails (Acha and Szyfres, 2003).
The principles of isolation and quarantine should be kept in mind while placing animals in lab animal colonies, farms, or feedlots. This involves creating flow patterns that reduce the amount of contact between vulnerable, healthy animals and sources of infection. The idea of a “closed herd” involves the use of quarantine (Acha and Szyfres, 2003).
Programmes for population control are additional strategies for lowering contact. The main goals of leash rules are to prevent rabies and lessen faecal pollution. Although leash regulations seem reasonable in theory, public indifference, if not outright resistance, and a lack of enforcement have made them ineffective (McQuiston and Childs, 2002).
- Increasing Host Resistance:
In addition to neutralization of reservoirs or contact reduction, zoonoses can be controlled by increasing host resistance to infection. The best course of action is to prevent infection, but in many cases, strengthening host resistance may merely moderate the severity of the illness without also strengthening resistance against infection (Martinma, 2007). However, chemoprophylaxis and immunisation are the two methods for boosting host resistance that are suitable for presentation. (Beers et al., 2006).
- Chemoprophylaxis:
Chemoprophylaxis aims to stop infection or lessen the illness’s severity, at the very least. Unlike immunisation, it is a passive method that only works as long as the medication does. On the other hand, an immunization’s active response might persist for months or even a lifetime. Usually, there is no reaction from the host. One example of the incorrect form of chemoprophylaxis, or no infection and no immunity, is the live anthrax vaccination given concurrently with penicillin prophylaxis (Jones et al., 2006). Chemoprophylaxis is usually used in the absence of any other, more potent host protection strategy. People’s antimalarial drug is one example. By inhibiting the erythrocytic stage of the malaria parasite, the drug lessens the severity of the disease’s effects but does not prevent infection (the mosquito injects sporozoites) (Berekeet,2008). Slow- or pulse-release boluses of oxfendazole have been shown in veterinary medicine to be beneficial in lowering pasture contamination and parasite gastroenteritis in cows. Among the most popular chemoprophylactic products for household animals are dog heartworm prevention pills and insect repellents that block arthropod vectors (Jones et al., 2006).
6.2 Immunization:
In addition to shielding vulnerable people from illness or infection, vaccines also stop the spread of infectious agents by building an immune system within the population. The stimulation of immunisation should be adequate to prevent both infection and disease in order to be most successful in reducing disease. If illness is the sole thing avoided in maintenance hosts, there is no decrease in the reservoir of infection. When comparing the percentage of the population that develops the necessary degree of protection to the resources used (vaccine, equipment, labour, promotion, etc.), the effectiveness of immunisation as a disease control strategy is evaluated (Ibrahim,2010). Immunisation is a normally very effective way to prevent disease; nonetheless, there can be delivery system failures, immune response failures, or iatrogenic errors that cause the failures (Jones et al., 2006).
- Animal assisted interventions:
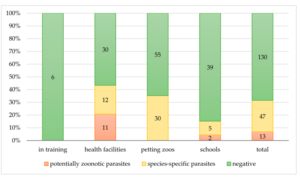
The purpose of AAIs is to expose stakeholders to possible infection risk. To ensure public health, zoonotic pathogen surveillance is required. This study looked into the existence of dermatophytes and other potentially zoonotic parasites in animals implicated in AAIs. AAIs may act as a direct cause of infection or as a result of environmental pollution. Active surveillance is therefore required, and the timing and planning of animal screenings should take the risk of exposure into consideration (Simonato et al., 2020). The study was conducted by simonato and coworkers on zoonoses linked to animal assisted interventions.
Figure 1: Number of study animals and their distribution in the main settings of animal-assisted interventions (AAIs) (Simonato et al., 2020).
Figure 2: Copromicroscopic results. Distribution of negative and positive (zoonotic and species-specific) results among the contexts of the AAIs (Simonato et al., 2020).
Figure 3: Copromicroscopic results. Distribution of negative and positive (zoonotic or species-specific) results in each animal species (Simonato et al., 2020).
Given the well-documented benefits of AAIs for humans and the growing national expansion of animal-related activities, it is important to consider the potential danger of pathogen exposure for both humans and animals. The gathered information emphasises the necessity of developing standards and instruments for evaluating the welfare and health of animals working in AAIs, taking into account generally asymptomatic animals that may have possible zoonotic infections. According to the increasingly significant idea of “One Health,” various protocols should be designed based on the actual risk of animal exposure to pathogens in relation to the lifestyle, management, and type of AAIs in which they are involved in order to guarantee the health of both humans and animals (Simonato et al., 2020).
- CONCLUSION:
To achieve one world and one health for prevention and control of zoonoses it is better to know the etiology of bacterial, fungal, viral, protozoal and parasitic zoonoses, host diversity and symptoms needs to be known for effective diagnosis and treatment and their strategies for eradication, control and preventive measures like reservoir neutralization, reducing contact potential, quarantine, chaemoprophylaxis, immunization needs to be followed and preliminary survey in animals involved in AAIs represents a starting point for further investigation.
- REFERENCES:
Acha P, Szyfres B (2003) Zoonoses and communicable disease common to man and animals Volume 2: chlamydioses, rickettsioses and viruses. (3rd edn), Pan American Health Organization, Washington, USA.
Beers MA, Porter RS, Jones TV, Kaplan JL, Berkwits M (2006) The Merck Bailleir Tindall, London, UK, pp. 809.
Bereket T (2008) Prevalence and economic impact of bovine hydatidosis at AA abattoir, DVM Thesis, FVM, DZ, Ethiopia. P. 12.
CDC. One Health. Available online: https://www.cdc.gov/onehealth/index.html (accessed on 27 April 2020).
Chomel, B.B. Zoonoses. In Encyclopedia of Microbiology, 3rd ed.; Elsevier Inc., University of California: Davis, CA, USA, 2009; pp. 820–829.
Gerardi, F.; Santaniello, A.; Del Prete, L.; Maurelli, M.P.; Menna, L.F.; Rinaldi, L. Parasitic infections in dogs involved in animal-assisted interventions. Ital. J. Anim. Sci. 2018, 17, 269–272.
He, J., Guo, Z., Yang, P., Cao, C., Xu, J., Zhou, X., & Li, S. (2022). Social insights on the implementation of One Health in zoonosis prevention and control: a scoping review. Infectious Diseases of Poverty, 11(03), 1-11.
Hubálek, Z. Emerging human infectious diseases: Anthroponoses, zoonoses, and sapronoses. Emerg. Infect. Dis. 2003, 9, 403–404.
Ibrahim MM (2010) Study of cystic echinococcosis in slaughtered animals in Al Baha region, SaudiArabia: interaction between some biotic and abiotic factors. Acta Tropica, 113(1): 26-33.
Italian National Guidelines in Animal Assisted Interventions. 2015. Available online: http://www.salute.gov. it/imgs/C_17_opuscoliPoster_276_allegato.pdf (accessed on 2 October 2020).
Jones TC, Hunt RC, King NW (2006) Veterinary Pathology. (6th Edn), Blackwell Publishing, USA, pp. 655-656.
Martinma EA (2007) Oxford Concise Medical Dictionary. (7th Edn), Oxford University Press, Bungar, UK pp. 342-343.
McQuiston J, Childs J (2002) Q Fever in human and animals in the United State, Vector Borne Zoonotic Dis 2: 179-191.
Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet (Lond.) 2012, 380, 1956–1965.
Olayemi, A.; Adesina, A.S.; Strecker, T.; Magassouba, N.F.; Fichet-Calvet, E. Determining Ancestry between Rodent-and Human-Derived Virus Sequences in Endemic Foci: Towards a More Integral Molecular Epidemiology of Lassa Fever within West Africa. Biology 2020, 9, 26.
Rahman, M. T., Sobur, M. A., Islam, M. S., Ievy, S., Hossain, M. J., El Zowalaty, M. E., … & Ashour, H. M. (2020). Zoonotic diseases: etiology, impact, and control. Microorganisms, 8(9), 1405.
Schellenberg R, Tan B, Irvine J, Stockdale R, Gajadhar A, et al. (2003) An outbreak of trichinellids due to consumption of bear meat infected with Trichinella nativa, in 2 northern Saskatchewan communities. J Infect Dis 188(6): 835-843.
Simonato, G., Danesi, P., Frangipane di Regalbono, A., Dotto, G., Tessarin, C., Pietrobelli, M., & Pasotto, D. (2020). Surveillance of zoonotic parasites in animals involved in animal-assisted interventions (AAIs). International journal of environmental research and public health, 17(21), 7914.
Sohn H, Probert W, Glaser C, Gupta N, Bollen W, et al. (2003) Human neuro brucellosis with intracerebral granuloma caused by a marine mammal Brucella spp. Emerg Infect Dis 9(4): 485-488.
Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989.
UNIDO. Coronavirus: The Economic Impact. 2020. Available online: https://www.unido.org/stories/ coronavirus-economic-impact (accessed on 23 May 2020).
World Health Organization. WHO Health Topic Page: Zoonoses. Available online: https://www.who.int/ topics/zoonoses/en/ (accessed on 20 July 2020).