Role of the Veterinarians and One Health in the Fight Against Zoonoses
Gadewar S. S.1, Deshmukh V. V.2
College of Veterinary and Animal Sciences, Parbhani (MAFSU, Nagpur)
- M.V.Sc. Scholar, Dept. of Veterinary Public Health and Epidemiology, College of Veterinary and Animal Sciences, Parbhani. Email ID- shridhargadewar@gmail.com
- Professor, Dept. of Veterinary Public Health and Epidemiology, College of Veterinary and Animal Sciences, Parbhani.
Introduction to Zoonoses
The link among humans, animal population and the surrounding environment is very close in many developing countries, where animals provide transportation, draught power, fuel, clothing and source of protein (that is meat, eggs, and milk). In the absence of proper care, this linkage can lead to a serious risk to public health with huge economic consequences .
Zoonoses are defined as those diseases and infection naturally transmitted between people and vertebrate animals . Zoonoses constitute a diverse group of viral, bacterial, rickettsial, fungal, parasitic and prion disease with a variety of animal reservoir, including wildlife, livestock, pet animals and birds . The transmission may occur through direct contact with the animal, through vectors (such as fleas or tick), or through food or water contamination. Globally zoonoses are said to account for 60% of all infectious disease pathogens and 75% of all emerging pathogens . In both developing and developed countries, several new zoonoses have emerged. This might be the result of either newly discovered pathogens or agents that are already known, usually appearing in animal species in which the disease had not previously been detected . Many diseases that affect human which are new, emerging and re-emerging were caused by pathogens that originated from animal. Moreover, several zoonotic diseases including rabies, brucellosis, bovine tuberculosis and echinococcosis continue to affect human and animals in many countries, particularly developing nations .
It has been observed that 75% of emerging pathogens fall within the category of zoonotic disease. Zoonotic disease caused mortality and morbidity in people, while also imposing significant economic losses in the livestock sector. They have both direct and indirect effects on livestock health and production . Indirect effects occur as a result of the risk of human disease, the economic impact on livestock producers through barriers to trade, the costs associated with control programmers, the increased cost of marketing produce to ensure it is safe for human consumption, and the loss of markets because of decreased consumer confidence .
The History and Introduction to One Health:
The One Health concept is not new, though it has been rebranded several times. Its origin lies in comparative medicine, the idea that there is no line between humans and animals when it comes to health and disease. When founding the first veterinary school in Lyon, France in 1761, Claude Bourgelat emphasized the importance of comparative biopathology. Later, Rudolph Virchow, William Osler, and John McFaydean carried the concept forward by incorporating veterinary perspectives into human health care through their respective work in comparative medicine, veterinary pathology, microbiology, and veterinary and medical education . Schwabe and Steele used the term “One World, One Medicine, One Health” to refer to their transdisciplinary work . While the pioneers of the One Health concept recognized that environmental factors played a crucial role in the well-being of humans and animals, the value of environmental health for the benefit of the ecosystem itself was not emphasized .
Many have their own definition of One Health, but the common thread is collaboration on a global scale among multiple disciplines to ensure the health of humans, domestic animals, and the ecosystem (including wildlife) in the industrialized and developing worlds . This forms a triad of health specialties, functions, and activities (Fig. 1).
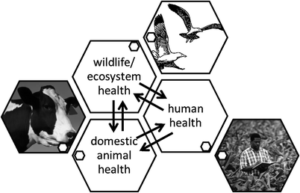
The Historical Role of the Veterinary Profession in One Health
In contrast with human medicine, the role of the veterinarian in society has greatly expanded since the founding of the first veterinary school in Lyon in 1761. The school was primarily established to combat an epidemic of rinderpest, the most feared disease of cattle in that era that was ravaging France . Following the establishment of the Lyon veterinary school, veterinary schools were soon opened in other countries around the world . Throughout the nineteenth century and the early part of the twentieth century, the focus of veterinary education in these schools was on training veterinarians to control disease in food producing animals, to prevent the transmission of zoonotic diseases, and, importantly, on the clinical care of the horse (riding, draft, and warfare).
Table 1 outlines the range of responsibilities of veterinarians with regard to One Health.
Table 1:
Roles and responsibilities of veterinarians in One Health
| Human health | Domestic animal health | Ecological health |
| Reduce global hunger | Promote animal welfare | Protect biodiversity |
| Control zoonoses | Prevent disease outbreaks | Management of wildlife resources |
| Monitor food quality and safety | Increase domestic animal production for food | Control movement of exotic species and diseases |
| Biomedical research | Increase and support animal product exports | Disease prevention in wild animal populations |
| Disease surveillance | Disease surveillance, diagnosis, and control | Disease surveillance |
| Biosecurity | Provide clinical and population health expertise for all animals | Conservation of natural resources, conservation medicine |
| Human–animal bond: maintaining companion animal health | Combatting antimicrobial resistance | Climate change adaptation activities |
Role of the Veterinarians and One Health in the Fight Against Zoonoses:
- Responding to Emerging Disease Outbreaks Caused by Zoonotic Agents:
The contributions of veterinarians to the One Health concept are most easily appreciated, when one examines the response to the emergence of a disease either for the first time or in a new environment. It is in this setting when a disease is known to be zoonotic (or suspected to be zoonotic), that the interdisciplinary approach is most readily visible. This “reactive” role of veterinarians in support of One Health can be categorized as Direct or Indirect.
The Direct Approach to Disease Investigation and Control is exemplified by multidisciplinary teams which come together in the field to work side-by-side in solving an emerging disease problem. This occurred in response to the early outbreaks of Ebola and Marburg virus infections in Africa, with teams of veterinarians, physicians, epidemiologists, wildlife experts, entomologists, and anthropologists physically working in the field .
The response to the introduction of West Nile virus into North America is a second example of a reactive multidisciplinary response. Concurrent human and avian encephalitis outbreaks in New York City, New York, USA in 1999 resulted in a joint effort by physicians and veterinarians around the world to determine its cause and source . Meanwhile, the race was on to produce an effective vaccine to prevent mortalities in the pleasure, racing, and working equine populations . One such vaccine, which is now commercially available for horses, was developed in parallel with a similar West Nile vaccine for humans .
The Indirect Approach to Disease Investigation and Control occurs when veterinarians work on a component of a One Health problem and share the results though information exchange. This approach is commonly seen once the causative agent has been identified and in the later stages of an outbreak or epidemic. Research is an important component; the veterinarians provide a piece of the puzzle, encouraging scientists in other fields to build on it and advance to the next research step. Surveillance of highly pathogenic H5N1 avian influenza virus circulation in the field (humans, poultry, and wild birds) and molecular research on the virus conducted in the laboratory serve as an example of the indirect approach.
Most disease outbreaks/epidemics are investigated by a combination of the direct and indirect approaches with several types of multidisciplinary teams being assembled for different aspects of the investigation. This is probably the most common approach to practicing One Health today. The individual teams may represent only part of the One Health triad, such as veterinarians working with physicians and microbiologists on vaccine development, or veterinarians engaging wildlife biologists and entomologists to determine the ecology of disease reservoirs and vectors. The control of bovine tuberculosis in cattle and badgers in the UK and Nipah infections in South East Asia are two excellent examples of this approach .
- Prevention of known zoonotic diseases:
While the One Health “reactive” approach to outbreaks of disease, as described above, attracts great attention from scientists, the public, and government, the major contribution of veterinarians to One Health lies in their day-to-day routine activities. These activities can be regarded as “proactive” One Health. In this context, their contributions to the multidisciplinary team approach are indirect. The production of a safe and reliable source of food from “farm to fork” involves thousands of veterinarians around the world; the human–animal interface may not be obvious, but it exists. From clinical treatment of individual animals using the appropriate antibiotic on the farm to zoonotic disease surveillance activities in free-ranging wildlife, One Health is being practiced each and every day. Even the daily activity of a veterinarian vaccinating a dog against rabies is One Health in action, even though it may not be recognized as such.
- Pathogen Discovery of Potential Zoonotic Agents
The spate of viral diseases that emerged in the first decade of this century which focused the need for One Health in the control of emerging diseases also drew attention to the importance of reservoir species, particularly wildlife, as a source of epidemic disease in humans . Identifying potential agents in wildlife species that are capable of “jumping species” to cause disease in humans and domestic animals is a difficult task and falls within the arena of ecosystem and wildlife health. Veterinarians are in the forefront in this field which has become known as “pathogen discovery”. A good example of such a program is PREDICT, a global early warning system for emerging diseases supported by the United States Agency for International Development (USAID) within its Emerging Pandemic Threats Program (http://www.vetmed.ucdavis.edu/ohi/predict/index.cfm). The program has established a global early warning system to detect and reduce the impacts of zoonotic diseases that emerge from wildlife.
- Some Specific Actions
- The chaos in the health services and consequently, in economic systems worldwide due to COVID-19 was at times the result of disobedience and non-compliance with the guidelines of preventive medicine, which outline the best strategies for containing the spread of the disease. Veterinarians have experience in successfully managing outbreaks of diseases, such as brucellosis, tuberculosis, anthrax, foot-and-mouth disease, and rabies, in addition to controlling zoonotic pathogens in foods of animal origin . Control measures, when strictly applied to animals, have resulted in a significant reduction of zoonoses in humans. Some of the specific actions of the veterinary profession are shown in Figure 2.
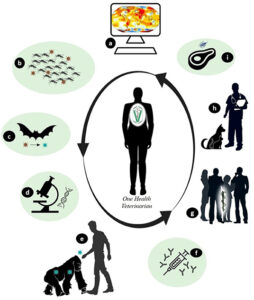
Figure 2. Examples of specific actions by the veterinarians to combat the emergence of zoonotic diseases. (A) – Studies to monitor multiple factors (environmental, temporal, and others) available in Geographic Information Systems that allow indicating changes for the modeling and forecasting of diseases. (B) – Environmental control that indirectly interferes in the control of vectors and/or hosts amplifying agents that transmit zoonotic diseases. (C) – Monitoring of wildlife through research that evaluates changes in the host and potential pathogens. (D) – In vitro investigation regarding the evolution of the characteristics of infectious agents over time. (E)-Health education through clarification to the population about the risks and care to be taken in human-animal contact. (F) – Production of diagnostic tests and vaccines using animal models based on comparative medicine. (G) – Application of translational medicine and zoobiquity in the exchange of experiences between teams of multi health professionals. (H) – Sanitary control in pets and production animals. (I) – Inspection and control of food of animal origin.
- Surveillance measures represent the main strategy, considering these preventive needs. Active surveillance is important in the investigation of the potential pathogens of animals and the potentials of possible emergence in humans. This type of control would allow the acquisition of rich databases that would support specific and effective measures to control zoonotic epidemics. High-risk behaviours could also be identified, and health education activities could be initiated to change habits that contribute to and hinder the adaptation and dissemination of the pathogens.
- Veterinarians are especially important in wildlife surveillance, which becomes a fundamental parameter in the control of emerging zoonoses because ecological changes, molecular variations of infectious agents, and wild animal-man interactions represent the main factors for the emergence of new pathogens. Therefore, the collaboration between veterinary communities linked to the monitoring of wildlife and human medical communities is crucial in the development of preventive strategies and must follow a double direction in the provision of early and specific information, which is not evident in most developing countries.
- Microbiology studies combined with physiology, immunology, and behavioural ecology must be applied to asymptomatic animal hosts. They can demonstrate which mechanisms could explain the absence of clinical signs and can provide effective and applicable responses to humans. At the same time, they are specific animal models, which provide ideal conditions for the reproduction of the disease showing similarity to human responses and should be used to carry out vaccine and treatment tests before being applied to humans. Besides, prior knowledge of the etiologic agent, its intrinsic characteristics, and ecology can facilitate the faster development of vaccines and therapy drugs in cases of emergency and health risk.
- Comparative medicine, in addition to in vitroanalyses, also covers field studies in the prevention of zoonosis. A good example of this is the monitoring of diseases that occur naturally in animal populations that can signal potential threats to human health. The double meaning in interventions must be attributed to the One Health approach considering the risks shared between humans and animals.
- Veterinary epidemiology allows alignment with disease forecasting and modeling studies through the application of georeferencing software that associates environmental variables, such as temperature, humidity, soil type, vector density, pathogen, host, exposure, and transit of animals and people. The convergence of factors that include the availability of these geocoded multi-temporal data and multi-professional collaborations worldwide would allow for the production of a sophisticated Geographic Information System under a holistic perspective for the development of research related to the control of zoonosis.
- Several recent studies have highlighted the fundamental importance of the veterinarian’s performance in the context of One Health. van Doremalen et al. have demonstrated the preliminary efficacy of a vaccine tested on mice and Rhesusmacaques against SARS-CoV-2 in partnership with a multi-professional team that includes veterinarians. In Chile, another multidisciplinary group, including veterinarians, developed an improved procedure to produce nanobodies using alpacas (Lama pacos) as donor species. The authors reported an optimized, fast, efficient, inexpensive, and simple density gradient method for nanobody selection and a sub-nanomolar affinity nanobody against the Spike receptor binding domain of SARS-CoV2. This proposed methodology may help in the generation of diagnostic and potentially therapeutic measures against COVID-19 and other infectious and emergent viruses . Sun et al. reported a variant of H1N1 from pigs with the greatest pathogenic potential in humans. Their experimental work was carried out in veterinary laboratories in the USA. In India, Dhama et al. have reported intense research performed with veterinarians in the development of vaccines and effective therapies against Ebola. Maki et al. reported that the monitoring of wildlife allowed the implementation of control strategies such as the use of bait vaccines to control wild rabies.
Conclusion:
Variations in behaviour and different human activities, such as the consumption and sale of wild animals, the poor application of food security rules, the advance of urbanization into rural areas, and constant direct contact with animal reservoirs are recognized as the main risk factors that lead to zoonoses outbreaks. All these factors can be tackled with preventive actions from a veterinary perspective, which are the resolutive competencies of this profession.
To avoid future emerging zoonoses, it is necessary to be prepared. The most effective way may be to maintain the natural barriers between animals that are reservoirs and human society, applying the conceptualization of the One Health doctrine in these actions. The veterinarian must assume a position of leadership in research and actions that primarily involve prevention and surveillance, which must be undertaken as an important part of maintaining public health, especially related to emerging and re-emerging zoonoses.
References:
- WHO (2010) Managing zoonotic public health risks at the human animal ecosystem interface Strong intra-sectoral partnerships in health, food safety and zoonoses?
- WHO (2005) The control of Neglected Zoonotic Diseases Report of a joint WHO/DFIDAHP Meeting with the participation of FAO and OIE? Geneva, Switzerland.
- Nkuchia M, Ruth L, Chris A, Henriette V (2007) Infectious diseases surveillance. Blackwell publishing Inc USA pp 246-248.
- WHO (2004) Water borne Zoonoses, Identification, Causes and Control.
- Jonathan R, Joshua L (2006) Emerging infectious diseases from the global to the local prospective. A summary of a workshop of the forum on Emerging infections. National Academy Press Washington, USA.
- Meslin F, Stohr K, Heymann D (2000) Public health implications of emerging zoonoses. Rev Sci Tech 19(1): 310-317.
- Smits H, Cutler S (2004) Contributions of biotechnology to the control and prevention of brucellosis in Africa. Afr J Biotechnol 3(12): 631-636.
- McDermott J, Arimi S (2002) Brucellosis in sub-Saharan Africa: epidemiology control and impact. Vet Microbiol 90(1-4): 11-134.
- Gibbs S.E.J., Gibbs E.P.J. (2012) The Historical, Present, and Future Role of Veterinarians in One Health. In: Mackenzie J., Jeggo M., Daszak P., Richt J. (eds) One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases. Current Topics in Microbiology and Immunology, vol 365. Springer, Berlin, Heidelberg.
- Monath TP, Kahn LH, Kaplan B (2010) Introduction: One Health perspective. ILAR J 51(3):193–198.
- Gibbs EP, Anderson TC (2009) One world-One Health and the global challenge of epidemic diseases of viral aetiology. Vet Ital 45(1):35–44.
- Okello AL, Gibbs EP, Vandersmissen A et al (2011) One Health and the neglected zoonoses: turning rhetoric into reality. Vet Rec 169(11):281–285.
- American Veterinary Medical Association (AVMA) (2012a). http://www.avma.org/reference/marketstats/usvets.asp. Accessed Mar 2012.
- American Veterinary Medical Association (AVMA) (2012b). http://www.avma.org/about_avma/whoweare/oath.asp. Accessed Mar 2012.
- Breman JG, Johnson KM, van der Groen G et al (1999) A search for Ebola virus in animals in the Democratic Republic of the Congo and Cameroon: ecologic, virologic, and serologic surveys, 1979–1980. J Infect Dis 179(Suppl 1):S139–S147.
- Lanciotti RS, Roehrig JT, Deubel V et al (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286(5448):2333–2337.
- Steele KE, Linn MJ, Schoepp RJ et al (2000) Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City. New York Vet Pathol 37(3):208–224.
- Monath TP (2001) Prospects for development of a vaccine against the West Nile virus. Ann N Y Acad Sci 951:1–12.
- Davis BS, Chang GJ, Cropp B et al (2001) West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol 75(9):4040–4047.
- Long MT, Gibbs EP, Mellencamp MW et al (2007) Efficacy, duration, and onset of immunogenicity of a West Nile virus vaccine, live Flavivirus chimera, in horses with a clinical disease challenge model. Equine Vet J 39(6):491–497.
- Wilson GJ, Carter SP, Delahay RJ (2011) Advances and prospects for management of TB transmission between badgers and cattle. Vet Microbiol 151(1–2):43–50.
- Pulliam JR, Epstein JH, Dushoff J et al (2012) Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface 9(66):89–101.
- Gibbs EP (2005) Emerging zoonotic epidemics in the interconnected global community. Vet Rec 157(22):673–679.
- Neerukonda SN, Katneni U. A review on SARS-CoV-2 virology, pathophysiology, animal models, and anti-viral interventions. Pathogens.(2020) 9:426. doi: 10.3390/pathogens9060426.
- Lapiz SM, Miranda ME, Garcia RG, Daguro LI, Paman MD, Madrinan FP, et al. Implementation of an intersectoral program to eliminate human and canine rabies: the Bohol Rabies Prevention and Elimination Project. PLoS Negl Trop Dis. (2012) 6:e1891. doi: 10.1371/journal.pntd.0001891.
- Peck D, Bruce M. The economic efficiency and equity of government policies on brucellosis: comparative insights from Albania and the United States of America. Rev Sci Tech. (2017) 36:291–302. doi: 10.20506/rst.36.1.2629.
- Verteramo CLJ, Tauer LW, Smith RL, Grohn YT. Assessment of the bovine tuberculosis elimination protocol in the United States. J Dairy Sci. (2019) 102:2384–400. doi: 10.3168/jds.2018-14990.
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv [Preprint].(2020). doi: 10.1101/2020.05.13.093195.
- Nieto GV, Jara R, Himelreichs J, Salinas C, Pinto T, Cheuquemilla Y, et al. Fast isolation of sub-nanomolar affinity alpaca nanobody against the Spike RBD of SARS-CoV-2 by combining bacterial display and a simple single-step density gradient selection. BioRxiv(2020). doi: 10.1101/2020.06.09.137935.
- Sun H, Xiao Y, Liu J, Wang D, Li F, Wang C, et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc Natl Acad Sci USA. (2020) 117:17204–10. doi: 10.1073/pnas.1921186117.
- Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, et al. Advances in designing and developing vaccines, drugs, and therapies to counter ebola virus. Front Immunol. (2018) 9:1803. doi: 10.3389/fimmu.2018.01803.
- Maki J, Guiot AL, Aubert M, Brochier B, Cliquet F, Hanlon CA, et al. Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): a global review. Vet Res.(2017) 48:57. doi: 10.1186/s13567-017-0459-9.
- https://www.pashudhanpraharee.com/role-of-veterinarians-and-one-health-in-the-fight-against-zoonoses-3/#:~:text=ROLE%20OF%20VETERINARIANS%20AND%


