RUMINANT KETOSIS: WITH REFERENCE TO KETONE METABOLISM
BY- HARNEET KOUR1, OLYMPICA SARMA2
Department of Animal Nutrition, Department of Animal Genetics and Breeding,
GADVASU, Ludhiana
1MVSc. Scholar, Department of Animal Nutrition,
GADVASU, Ludhiana, Punjab, 141004,
E-mail: Harneetkour178@gmail.com
2MVSc. Scholar, Department of Animal Genetics and Breeding,
GADVASU, Ludhiana, Punjab, 141004,
E-mail: olylucky15@gmail.com
Introduction:
Ketosis is a metabolic disease that occurs when energy demands (e.g. high milk production) exceed energy intake and result in a negative energy balance, usually due to impaired metabolism of carbohydrates and volatile fatty acids. In pregnant and lactating animals, there is a continuous demand for glucose and amino acids and ketosis results when fat metabolism, which occurs in response to the increased energy demands becomes excessive (Williamson 2013). The free fatty acids released from fat metabolism are esterified into fatty acyl CoA in the liver. When fatty acids undergo excessive oxidation in the liver due to increased energy demands, large quantities of keto acids are produced — acetoacetic acid and β-hydroxybutyric acid, which pass into the blood by diffusion. Acetoacetic acid then undergoes spontaneous decarboxylation to produce acetone. These three substances—Acetoacetate, β- hydroxybutyrate and Acetone are collectively known as the ketone bodies (or acetone bodies).
Normally, the blood of mammals contains ketone bodies not exceeding 1 mg/100 ml. The concentration is little higher than this in ruminants. Daily excretion of ketone bodies of normal person is less than 1 mg (Taneja 2016). Higher than normal quantities within the blood or urine constitute ketonemia (hyper-ketonemia) or ketonuria, respectively. The condition during which there’s a high concentration of ketone bodies in tissues and blood is named ketosis.
Types of ketosis:
- On the basis of time of occurrence and pathology of disease:
- Type I ketosis: occurs at 4–6 week postpartum, may be more closely associated with underfed cattle experiencing a metabolic shortage of gluconeogenic precursors than with excessive fat mobilization.
- Type II ketosis: ketosis in the immediate postpartum period or in very early lactation are usually associated with fatty liver.
- On the basis of severity ketosis is of two types:
- Clinical ketosis: having a beta-hydroxy butyrate (BHB) blood level of ≥3.0 mmol/l (31.2 mg/d) and generally affects up to 15% of cows.
- Sub-clinical ketosis: begins at ≥1.2 mmol/l (12.4 mg/dl) levels of BHB and shows a prevalence of over 40% of cows in contemporary commercial herds.
Etiology and Pathogenesis:
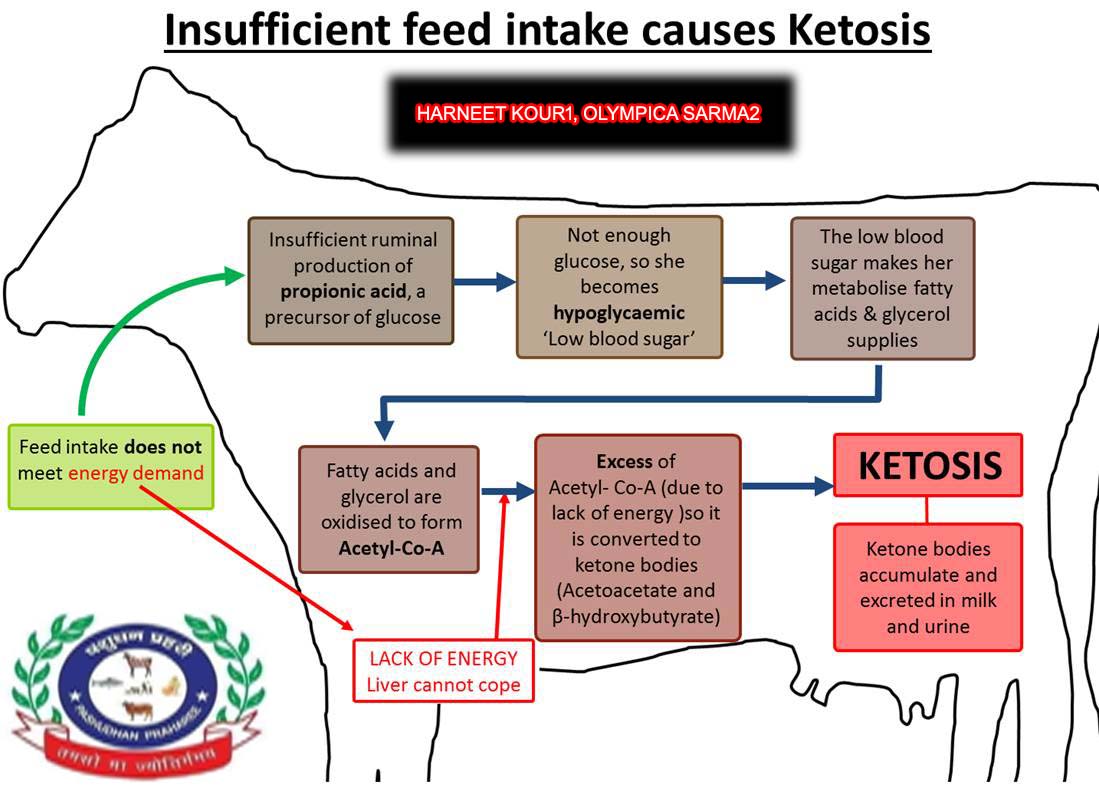
The pathogenesis of bovine ketosis is incompletely understood but is found to be associated with 2 phenomena i.e. intense adipose mobilization and a high glucose demand. Both of these conditions are present in early lactation. Negative energy balance during such time leads to adipose mobilization and milk synthesis creates a high glucose demand. Sometimes, glucose is present in blood but cells are unable to ultilize it as in case of Diabetes mellitus or type 1 diabetes. Adipose mobilization is accompanied by high blood serum concentrations of nonesterified fatty acids (NEFAs). Ideally NEFA gets converted to glucose via gluconeogenesis but during periods of intense gluconeogenesis, a large portion of serum NEFAs is directed to ketone body synthesis in the liver (Nazir 2018). Thus, the clinicopathologic characterization of ketosis includes high serum concentrations of NEFAs and ketone bodies and low concentrations of glucose (Herdt 2014).
The level of ketone bodies present in blood or milk are also correlated to increased risk for a number of metabolic disorders, including metritis, mastitis, left-displaced abomasum, all of which contribute to lower milk production and poor reproductive performance. Moreover, clinical ketosis preludes fatty liver syndrome, whereby circulating lipids that are not metabolized are deposited within the liver, resulting in further reduction in metabolic capacity and increasing risk of subsequent metabolic disorders.
Factors Contributing to Ketosis Development:
1. Body condition
The leading cause for the predisposition of ketosis development is excess body condition prior to calving. During transition tissue mobilization is predominantly from fat reserves; however, muscle tissues are also mobilized. The proportion of fat or muscle tissues mobilized depends on the body condition score (BCS) of the cow, with those having higher BCS mobilizing more body fat, more rapidly (Pires et al 2013) For this reason, cows slightly below the ideal body score are at less risk of developing ketosis than those with higher body scores.
2. Low feed intake
In the week prior to calving, there is a generally a sharp drop in DMI that induces fat mobilization and a corresponding rise in circulating non-esterified fatty acids (NEFA) to support the energy needs of the unborn neonate. Averting low DMI through diet management is vital to sub-clinical ketosis and subsequent metabolic disease prevention. Post-calving, cows that have marked lower intake than cohorts in a group are at a way higher risk of developing ketosis than those that are more aggressive eaters. Moreover, cows with low DMI tend to be more selective, thus increasing the risk of developing SARA.
3. Lameness
Lameness during the dry period can have a lasting effect into the fresh period as lameness affects cow comfort. Even mild lameness can increase laying time and disrupt eating patterns. This features an immediate effect on daily DMI and energy consumption, increasing the pressure on body fat reserves in early lactation inducing sub-clinical or clinical ketosis (Goldhawk 2009).
4. Hypocalcemia
Lower circulating calcium levels can affect muscle contraction, including rumen mobility. If rumen mobility is compromised, absorption of volatile fatty acids from fermentation of feedstuffs is reduced, limiting the potential for glucose formation. Low circulating glucose affects insulin levels, resulting in increased fat mobilization and heightening the risk of developing ketosis or fatty liver syndrome.
- Type 1 or Insulin dependent diabetes mellitus
The major example of pathological ketosis is insulin-dependent or type 1 diabetes. Insulin is absent or very low in the plasma and therefore there is no antagonistic action to restrain the opposing hormones, adrenaline, noradrenaline and glucagon. Consequently, lipolysis in adipose tissue is greatly stimulated and plasma fatty acids increase to high levels.
Ketone Body Metabolosim
Acetoacetic acid and β-hydroxybutyric acid are moderately strong acids and are buffered in blood or tissues. Their excretion in large quantities admits some loss of buffer cation which depletes the alkali reserve causing ketoacidosis. The process of formation of ketone bodies is termed Ketogenesis occurring in liver and the process of breakdown of ketone bodies taking place in peripheral tissues is called Ketolysis.
KETOGENESIS:
- First Pathway (Minor Pathway):
Fig 1. Minor pathway for ketogensis in liver
The first pathway is by simple deacylation catalyzed by the enzyme Acetoacetyl-CoA deacylase which coverts Aceto acetyl CoA to Acetoacetate/ acetoacetic acid (Taneja 2016).
- The Second Pathway (Major Route):
It involves the condensation of acetoacetyl-CoA with another molecule of acetyl-CoA to form β-hydroxy-β-methyl glutaryl-CoA by HMG-CoA synthetase. HMG-CoA is splitted into acetoacetic acid and acetyl-CoA by HMG-CoA lyase present in mitochondria. From acetoacetic acid, acetone and β-hydroxybutyrate are formed (Taneja 2016).
Fig 2. Major pathway for ketogensis (Munoz et al 2011)
KETOLYSIS:
Acetoacetic acid and β-hydroxybutyric acids are carried from liver to extra hepatic tissues mainly kidney and muscle where they are oxidized for energy production after conversion to acetyl-CoA. The enzyme responsible for the activation of acetoacetate to acetoacetyl-CoA is absent from liver for which liver cannot utilize these acids. Two reactions take place in extra hepatic tissues for the activation of Acetoacetate to Acetoacetyl-CoA:
- In the first reaction: Succinyl-CoA reacts with acetoacetic acid in presence of the enzyme acetoacetate-succinyl-CoA transferase (Thiophorase) to form acetoacetyl-CoA and succinate.
- In the second reaction: Acetoacetate is activated by ATP in presence of CoA catalyzed by acetoacetyl-CoA synthetase to form acetoacetyl-CoA.
Several pathways have been proposed for the utilization of acetone:
- First Pathway: Acetone is converted to acetoacetate by reversal of decarboxylation.
- Second Pathway: Acetone is converted to propanediol which can form 1 carbon (formate) unit and 2 carbon (acetate) units. Most of the evidence suggests that ketonemia is due to increased production of ketone bodies by the liver rather than to a deficiency in their utilization by extra hepatic tissues.
Regulation of Ketone Body Metabolism:
Ketosis does not occur in vivo unless the concentration of circulating free fatty acids increases by the lipolysis of triacylglycerol in adipose tissue. Free fatty acids are the precursors of ketone bodies in the liver (Pankaja 2010). The factors regulating mobilization of free fatty acids from adipose tissue are important in controlling ketogenesis.
- The activity of carnitine palmitoyl transferase I in the outer mitochondrial membrane regulates the entry of long chain acyl groups into mitochondria prior to β-oxidation. Fatty acid oxidation is depressed in fed state since the enzyme activity is low but high in starvation when fatty acid oxidation increases (Taneja 2016).
- In fed state, there is the increase in concentration of malonyl-CoA which inhibits β-oxidation. In fed state, free fatty acids enter the liver cells in low concentrations and all are esterified to acylglycerols and transported out of the liver in VLDL (Taneja 2016).
- When the concentration of serum free fatty acids is raised, more free fatty acid is converted to ketone bodies and less is oxidized via the citric acid cycle to CO2.
- The complete oxidation of one mole of palmitate produces 129 mol of ATP via β-oxidation and CO2production in the citric acid cycle, whereas only 33 mol of ATP is produced when acetoacetate is the end product and only 21 mole when 3-hydroxybutyrate is the end product.
Effect of Ketosis on Ruminants:
1. Metabolic acidosis: Both aceto-acetate and b-hydroxybutyrate are moderately strong acids. They neutralize bicarbonates resulting depletion of alkali of the body and produce metabolic acidosis. In case of severe ketosis, death may ensure from acidosis.
- Loss of electrolytes: The excretion of ketone body in the urine involves the loss of Na+in particular, leading to total electrolyte and Na+
- Dehydration: The severe diabetic patient excretes large quantities of both ketone bodies and glucose in the urine with a large quantity of water and thus developing dehydration. In diabetic acidosis, there is severe alteration in cation-anion balance in the plasma.
Metabolic diseases associated with Ketosis:
1. Displaced Abomasum:
The growth of the fetus during the last phase of the pregnancy tends to limit rumen volume, which may also contribute to declining intake leading up to calving. Cows in the sub-clinical ketosis risk range (BHB level 1.2 mmol to 3 mmol) have an eight times higher risk of developing left-displaced abomasum than those below the threshold level. Encouraging greater DMI post-calving greatly reduces the risk of developing left-displaced abomasum.
2. Lameness:
Lameness related to sub-clinical ketosis is often not apparent until later in the lactation. When sub-clinical ketosis disrupts eating behavior, leading to increased tissue mobilization and/or SARA conditions, the epithelial layer in the small intestine can be damaged, resulting in a condition known as “leaky-gut” syndrome. As the permeability of the small intestine is increased, small particulates, pathogenic bacteria and inflammatory proteins enter the cows’ circulatory system. Histamines in particular has been linked to inflammation in the claw, leading to weakened claw structure and bacterial infiltration. Cows in the sub-clinical ketosis risk range (BHB level 1.2 mmol to 3 mmol) have a 5 times higher risk of developing lameness than those below the threshold level.
3. Metritis:
Suppression of the cow’s immune system at calving increases the likelihood of developing metritis, such as when challenged by sub-clinical ketosis. Cows in the sub-clinical ketosis risk range (BHB level 1.2 mmol to 3 mmol) have a 2 times higher risk of developing metritis than those below the threshold level.
4. Low milk yield:
Milk yield is driven by lactose production, which is directly connected to liver function and glucose levels. Restricted glucose production in early lactation hinders the maturation of the milk secretion cells. The impact of ketosis on milk production depends on the severity. Conservative estimates from research suggest losses can range from 1 to 3 kg, which can exceed 350 kg lost production over the lactation (Rutherford 2016).
5. Poor fertility:
A voluntary waiting period of 60 days prior to first service is generally adequate, however, if suffering from sub-clinical or clinical ketosis, will delay ovulation or result in poor conception (Rutherford 2016).
Diagnosis:
1. Pre-calving symptoms
Metabolic tests indicate circulating NEFA levels above 0.30 mEq/L, or BHB levels of 0.6 to 0.8 mmol/L (≥ 6.25mg/dL) pre-calving put the cows at significantly higher risk of developing clinical ketosis in addition to metritis, retained placenta and displaced abomasums.
2. Post-calving symptoms
Circulating NEFA is a better indicator of metabolic disorder risk than BHB, in blood or milk. Cut-off values for NEFA risk of samples collected within the first 14 days are 0.6 to 0.7 mEq/L. Empirical cut-off levels of BHB are ≥1.2 mmol/l (12.4 mg/dl) for sub-clinical ketosis and ≥3.0 mmol/l (31.2 mg/d) for clinical ketosis.
- Clinical test
To properly assess the risk factors on individuals, ketosis testing should be performed at least twice between 3 and 14 days in milk. Alternatively, monitoring at the herd level, whereby 1 in 12 cows in early lactation testing above the 1.2 mmol/L cut-off, is indicative that ketosis is prevalent in the herd.
- Rothra’s test: Sodium nitroprusside tablets are used for detection of ketone bodies in urine.
- Ketosis dipsticks may be used to identify ketones in the urine or plasma.
- Blood glucose estimation: glucose less than 40 mg/dl, total blood ketones >30 mg/dl, and milk ketones >10 mg/dl is indicative of ketosis. In small ruminants, blood glucose levels found to be below 25 mg/dl and ketonuria are good diagnostic indicators.
- Visual signs
Often ketones can be smelled in the cow’s breath and milk. Rapid loss of BCS following calving is generally a strong indication of ketosis. Ideally, a cow will only lose 0.5 to 0,75 of a BCS, or roughly 40-65 kg of body weight. New technologies using 3D cameras are available to help monitor BCS. Low DMI relies on visual assessment of the cow’s rumen fill when viewed from behind. A cow that has been inadequately eating for more than a day will have a relatively straight belly line.
Treatment of Ketosis:
Treatment of ketosis is aimed at reestablishing normoglycemia and reducing serum ketone body concentrations (Herdt 2016).
- Bolus IV administration of 500 mL of 50% dextrose solution is a common therapy. This solution is very hyperosmotic, so given IV only.
- Bolus glucose therapy generally results in rapid recovery, especially in cases occurring near peak lactation (type I ketosis).
- Administration of glucocorticoids, including dexamethasoneor isoflupredone acetate at 5–20 mg/dose, IM, may result in a more sustained response, relative to glucose alone. Glucose and glucocorticoid therapy may be repeated daily as necessary (Herdt 2016).
- Propylene glycol administered orally (250–400 g/dose) once per day acts as a glucose precursor and is effective as ketosis therapy.
- A long-acting insulin preparation given IM at 150–200 IU/day may be beneficial in type 2 ketosis.
Prevention of Ketosis:
1. Encouraging greater DMI
It is the single most effective preventative measure against ketosis. Greater DMI enables similar energy intake, at a lower energy density, which allows the necessary effective fiber levels to maintain rumen function and metabolic processes. In comparison, increasing energy density is usually at the expense of effective fiber in exchange for higher soluble carbohydrates or fats content, leading to greater risk of rumen upset and SARA conditions.
2. Prophylactic treatment
Continual monitoring and early response to sub-clinical ketosis make prophylactic propylene glycol treatment a successful strategy to reduce the instances of clinical ketosis. The concept is to provide an amount of easily metabolizable glycerol that will increase circulating insulin levels sufficiently to reduce the mobilization of body fat and ease the metabolic pressure on the liver.
3. Feed additives
Feed additives that encourage greater DMI will help to reduce the instances of sub-clinical ketosis. Yeast supplements and phytogenic feed additives are commonly fed in dairy rations for their general effect of enhancing rumen buffering capacity and improving the degradation of feeds. This action stabilizes the rumen pH and microbiota, which in turn encourages greater mobility of the cow and more time spent at the feed bunk. To alleviate excess fat deposition in the liver, choline and methionine can be fed to help mobilize accumulated fat out of the liver where it can then be excreted into the milk.
- Feed bunk management
Bunk management of dry cows is often neglected as they are not considered to be productive animals, however, they too need adequate bunk space in order to ease social stress and maintain regular eating patterns.
5. In case of diabetic ketosis, carbohydrate diet, intramuscular injection of insulin and anti-ketogenic substance (aspartic acid) which may provide oxaloacetate by transamination should be administered.
- In case of prolonged starvation ketosis, carbohydrate diet and anti-ketogenic substance (aspartic acid) which may provide oxaloacetate by transamination should be given (Dhillon and Gupta 2020).
- The electrolytes and the fluids of the body must be restored by intravenous injection of isotonic solution of sodium salts such as NaCl, NaHCO3or sodium lactate. Potassium salts are desired to be added.
Conclusion:
Ketosis in animals occurs due to the increase of ketone bodies (Acetoacetate, β- hydroxybutyrate and Acetone) in the blood and tissue. Ketosis in ruminants is mostly associated with the negative energy balance. The animals suffering from ketosis normally have low blood glucose level and the primary cause of ketosis is decreased carbohydrates in the diet of the animal which will lead to low amount of glucose in the blood and ultimately which leads to fat metabolism and form ketone or acetone bodies. The drug of choice in ketosis is Propylene glycol and ketosis in ruminants can be prevented by increase in dry matter intake, providing adequate carbohydrate diet, yeast supplement, phytogenic feed additives etc. Since Ketosis is one of the important and most common metabolic disease in dairy animals therefore, proper treatment and management should be provided to the animals in order to prevent economic loss in dairy industry.
Reference:
- Arnedo M, Muñoz M R and Puisac B Mitochondrial. 2011. HMG-CoA Synthase Deficiency. Advances in the Study of Genetic Disorders pages:191-204.
- Dhillon K K and Gupta S. 2020. Biochemistry, Ketogenesis. National Center for Biotechnology Information, S. National Library of Medicine. StatPearls Publishing LLC.-4977.
- Goldhawk C, Chapinal N, Veira D M, Weary D M and von Keyserlingk M A G. 2009. Prepartum feeding behavior is an early indicator of subclinical ketosis. Journal of Dairy Science 92(10):4971
- Herdt H D. 2016. Overview of Ketosis in Cattle (Acetonemia, Ketonemia). MSDManual, Veterinary Manual.
- Kaneko J J, Harvey J W and Bruss M L. 2008. Lipids and ketones. Clinical biochemistry of domestic animals pages:81-116.
- Pankaja N. 2010. Chapter-13 Lipid Metabolism. Biochemistry
- Pires J A A, Delavaud C, Faulconnier Y, Pomiès D and Chilliard 2013. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. Journal of Dairy Science 96(10):6423-6439.
- Rutherford A J, Oikonomou G and Smith R. 2016. The effect of subclinical ketosis on activity at estrus and reproductive performance in dairy cattle. Journal of Dairy Science 99(6):4808-4815.
- Taneja A. 2016. Ketosis: Meaning, Regulation and Effects. Lipid metabolism, biology discussions.com.
- Williamson D H. 2013. Ketosis-an overview. Encyclopedia of Human Nutrition (Third Edition) Pages 47-53.