Significance of Somatic Cell Counts (SCC) in Milk and its Effect on Milk Parameters and Public Health
M.Afreed1*, T.Gokul1, M.Singh2,S.V.Singh2A.Saniyya2, C.Srividhya2
Department of Animal Physiology and Department of Animal Nutrition, National Dairy Research Institute, Karnal, Haryana, India
Abstract
Somatic cell counts (SCC) are widely used to predict the mammary health status of quarters and cows, the suitability of milk for human consumption & monetary losses to producers due to mastitis. Milk somatic cells are primarily leucocytes and some epithelial cells shed from lining of the mammary gland. Normal milk does contain cells and it is almost less than onelakh/ml in milk from uninfected/uninflammed mammary quarter. Research has clearly shown that environment or physiological factors such as lactation number, stage of lactation, estrus, mild exercise and estrus have marginal effects on SCC from uninfected (bacteriologically negative) quarters.
Introduction
Somatic cell counts (SCC) have been widely used as an indicator for monitoring the mammary health status of cows, the suitability of milk for human consumption and basis to provide incentives to the producers based on quality of milk. The term “Somatic cell count” refers to body-derived cells, frequently found in only trace amounts in milk.The milk SCC, which consists of leukocytes (neutrophils, lymphocytes, and macrophages) together with epithelial cells, plays a significant part in the udder’s natural defensive mechanism. Milk invitro leukocyte activity in can also be utilised to assess the udder’s health.(mukherjee et al,2013)
In a healthy quarter, the somatic cell population primarily consists of macrophages (approximately 60% of cells), lymphocytes (approximately 30%), polymorphonuclear (PMNs) cells (approximately 10%) and sloughed epithelial cells from lining of the mammary gland (Burvenich et al., 1995; Paape and Contreras, 1997). The macrophages, monocytes and PMNs constitute first line of defence once microorganisms have penetrated into the teat cistern. The PMNs are recruited to the site of infection by chemo attractant properties of cytokines and make up almost 90 percent of leukocytes present during infection in quarter. The PMNs mainly neutrophils use non-specific immune recognition system to bind ingest and destroy microorganisms through phagocytosis. Epithelial cells are more infrequent in udder secretions, including those from dry quarters and could range from 0 to 7 percent of the cell population (Lee et al., 1980). Thus increase in cell counts during infection are not due to sloughed epithelial cells, rather an influx of neutrophils into the milk comprises a major part of cells present early in inflammation (Miller and Paape, 1985). A number of other factors including management, stage of lactation, parity and season also influence SCC in milk (Dulin et al., 1983; Randy et al., 1991; Lin and Chang, 1994, Muggali, 1995; Singh and Ludri, 2001). Taking consideration of all the factors, SCC in milk can be used as a potential tool for monitoring udder health status and can eliminate the logistic and financial limitations of bacteriological culture for identifying intramammary infection (IMI).
Type of IMI and SCC
The most common organisms that infect the mammary gland can be divided into two groups: the major pathogens and minor pathogens. The major pathogens cause the greatest SCC increase and include Staphylococcus aureus, Streptococcus agalactiae, coliforms, and Streptococcus spp. other than Streptococcus agalactiae. The minor pathogens are Corynebacterium bovis and coagulasenegative Staphylococci. The major pathogens usually cause a 2- to 3-fold increase in SCC over that of uninfected quarters (Andrews et al., 1983; Reneau, 1986).
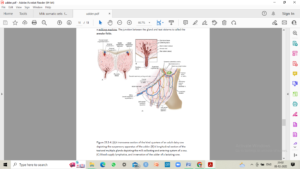
Fig 1: Diagrammatic representation of mammary gland anatomy
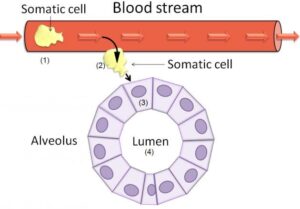
Fig 2: Schematic diagram of normal milk alveolus
Although the use of the cell count alone to classify quarters as infected or uninfected results in some degree of error due to false positives and false negatives (Dohoo and Meek, 1982). These errors may be due to the normal fluctuation of SCC observed throughout the course of an infection. Temporal changes in SCC following experimental challenge of mammary glands with various pathogens would suggest that there are dramatic changes in the magnitude of the SCC in the early stages of the infection (i.e. the acute phase), reaching a peak count within hours or days depending on the challenge organism. This may be followed by a modest reduction in SCC as bacteria are killed by neutrophils. The magnitude of decline in SCC can vary considerably and may be dependant upon the bacteriological outcome of the infection, the pathogen involved and cow differences. It is apparent that the SCC in infected quarters does not remain static but tends to fluctuate. In chronic infections SCC and bacterial numbers both tend to fluctuate up and down with time (Sears et al., 1990).
Fig 3: Diagrammatic representation showing both healthy and mastitis mammary gland.
The magnitude of SCC responses to major pathogens varies from cow to cow, and it does not seem possible to differentiate between the types of pathogens by SCC alone. Schultz (1977) reported that it might take days, weeks, or longer for SCC to decrease after the pathogens have been eliminated from the gland.
Sensitivity and Specificity of SCC for identifying IMI
The sensitivity can be calculated as the proportion of culture positive quarters that have SCC values above the selected threshold, and specificity is the proportion of culture negative quarters that have SCC values below the selected threshold (Martin et al., 1987). Sensitivity relates to the infected cows and specificity relates to the non– infected cows. The relation between these therefore changes in an inverse manner. Since the SCC in milk is a indirect test for defining IMI in a cow, it is necessary that critical value of SCC to distinguish infected or healthy quarter should optimize both sensitivity and specificity values. Earlier studies reported 70 to 90 percent sensitivity and 75 to 85 percent specificity with a SCC threshold of 200 x 103 cells/ml (Dohoo, 1990). Later, Kirk et al., (1996) found that the predictive value of increased SCC is better for the contagious or major pathogens compared with the environmental pathogens. In another study, Sargeant et al., (2001) reported maximum sensitivity (57.4) and specificity (72.3) with a SCC threshold >100 x 103 cells/ml for quarter samples taken on day five post-partum. Both sensitivity and specificity were again considerably higher for distinguish infection with major pathogens. Therefore, this screening test can be of great use for monitoring udder health programme particularly in dry cow management.
Importance of bulk tank SCC (BTSCC)
The major factor affecting SCC is an infection of the mammary gland. This holds true at the quarter, cow, or bulk tank level. Eberhart et al., (1982) studied infection prevalence in 80 herds and related this to bulk tank SCC (BTSCC) that ranged from 103,000 to 1,591,000 cells/ml. It is obvious that an increase in BTSCC is related to increased infection rate and decreased milk production. An analysis of these data showed that infection rate was the major determinant of BTSCC. An elevation above the 200,000/ml of milk is generally considered abnormal and an indication of inflammation in the udder. In well managed herd BTSCC should be within 1-2 lakhs/ml of milk.
One would also expect that the SCC in the bucket or composite milk would be related to the number of quarters infected and the amount of milk being produced by each. However, if all quarters of a cow were uninfected one would generally expect SCC below 200,000 in the bucket milk. BTSCC and herd average SCC score indicate the state of udder health in the herd and should be used to monitor trends and alert the dairy farmers to problems. The BTSCC is positively correlated with the number of quarter infected in the herd. Estimated infection prevalence and losses in milk production losses associated with elevated BTSCC .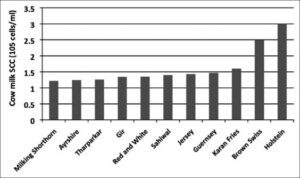
Fig 4: Milk somatic cell counts in different breeds of healthy cows. [Alhussien et al., 2018].
Somatic Cell Counts in Buffaloes
Various workers reported lower SCC of buffaloes compare to crossbred or local cows under similar set of managemental condition, which may be due to their thick streak canal and perfect closure mechanism of teat sphincter and the higher udder capacity of buffaloes leading to less stress of udder compared to the cattle. The total somatic cell count in normal buffalo milk varied from 50,000 to 3, 75,000 cells per ml and a variation were observed in the counts among the buffaloes. Neutrophil, the most frequently observed cell in buffalo milk, constituted an average of 56% (22-88%) of the total somatic cell counts. The second most commonly observed leucocyte in buffalo milk was lymphocyte, which constituted an average of 20% (10-54%) of the total somatic cell counts. The macrophages, epithelial cells and Eosinophils were 8%, 5% and 1%, respectively of the total somatic cell counts (Silva and Silva, 1994). It was estimated that the avg SCC of healthy quarter milk samples was 1.44 lakh/ml of milk and 1.11 lakhs/ml of milk in Sahiwal Cows and Murrah Buffaloes, respectively (Ghosh et al., 2004).
Effect of stress on SCC
Stresses of various types results in increases in SCC. Various form of stress like confinement of animals in hot condition; mammary pathogens and injections of ACTH could also lead to abnormality to high somatic cell counts in milk (Roussel et al., 1969 and Whittleston et al., 1970). Nelson et al. (1969) reported a positive relationship between high summer environmental temperature and SCC in milk. However, attempts to experimentally induce SCC changes in uninfected cows by injection of ACTH or corticosteroids or by placing animals in environmentally controlled chambers have shown only modest or no effects on milk SCC (Wegner et al., 1976). The high somatic cell counts observed in hot humid condition due to harsh climatic condition of high humidity and ambient temperature leading to stress condition and increase in susceptibility of infection (Doho and Meek, 1982; Hogan et al., 1989). Although a Florida study showed a significant increase in SCC in milk from heat-stressed cows, the respective mean SCC from cows (uninfected and infected with Staphylococcus spp.) subjected to either heat stress or housed in a thermoregulated environment were 145,000 and 105,000 (Elvinger et al., 1991). A portion of this difference in SCC may be due to the decreased milk yield that is observed under heat stress. It is not unusual to experience 10 to 20% decline in milk yield in dairy cattle experiencing heat stress (Shearer and Beede, 1990). Studies at NDRI (Singh, 2002) revealed that the hot humid season (July-August) increase in SCC of milk of dairy animals, mainly due to favorable environment for growth of bacteria. During hot dry season (May-June), SCC is moderate and is low in cold season (December-January) and when the stress on the udder of the cow is minimum. Better cooling strategies (Fan, SCC2.89 lakhs Vs. Fan and Sprinkler, SCC-2.21 lakhs/ml of milk) improves the udder health status of cow (Ghosh et al., 2007)
Milking systems and SCC
The milking machine is an essential part of the dairying industry, but it is assumed that its improper use has been the principal factor in the increase of sub-clinical (largely staphylococcal) mastitis in recent years. It affects udder health in several ways, such as carrying pathogens from one cow to next, serving to carry infection from one quarter to another in the same cow by permitting reflux of milk from one infected quarter back onto others, traumatizing the teat and thus predisposing the quarter to invasion by bacteria and allowing the passage of pathogens into the teat canal by an impact mechanism, created by an abrupt loss of milking vacuum pressure. Hence, fluctuation of vacuum has been found to be the most common fault of machine milking system in causation of mastitis. In addition to above, leaving the milking machine operative on teat after flow of milk has been ceased (over milking), which injure streak canal due to excess pressure and over pulsation also predispose animals to mastitis infection. Use of large cup in smaller teat, which causes injury to the teat and udder tissue, improper cleaning, disinfections and handling of machine are one of important cause to the higher occurrence of mastitis in machine-milked cows (Schalm et al., 1971). Machine milking could cause disturbances of circulation within bovine teat tissue, which impair its ability to withstand mastitis pathogens. Changes in teat end thickness can be caused as a measure of circulatory impairment due to congestion and edema, and SCC were always significantly greater with machine milking than calf suckling or hand milking (Hamann et al., 1991)
Impact of increased somatic cell counts in milk
Milk Production
This is perhaps the most important loss associated with higher SCC. A reduction in milk yield occurs even with mild infection and without any visible changes in milk. SCC levels of 0.6-1 x 106 cells/ml were found to cause a reduction of 8-12 percent milk production (Philpot, 1978). SCC level above 1 x 106 cells/ml milk reduced milk yield by over 900 kg per lactation (Miller and Paape, 1985). A bulk tank SCC analysis showed that SCC of 0.5, 1.0 and 1.5 x 106 cells/ml resulted in reduced milk yield at the rate of 1.5, 4.1 and 6.6 kg/day. Therefore, progress of any mastitis programme can be assessed by monitoring the herd average SCC as well as changes in herd average.
Milk Quality
In infected udder, compositional changes occur in milk due to altered membrane permeability, altered blood flow to the udder and changes in the filtering capacity. Higher SCC in milk is generally associated with low keeping quality, fat and casein content and higher bacterial counts (Philpot, 1978; Ghosh, 2004). The fat test has shown varying reduction as minimum as 4 percent (Asby et al., 1977) to a drastic reduction of about 11 percent (Everson, 1980). Solid not fat content showed a more obvious reduction from 11 to 15 percent (Asbyet al., 1977). The total milk protein content seems to be unaffected but proportion of various protein fractions showed a change with increased SCC. Plasmin and enzymes derived from somatic cells can cause extensive damage to casein. A significant decrease occurs in casein content, but content of whey protein increases with higher SCC level (Ghosh, 2004). Sometime total milk protein content may show a higher value with increased serum protein content (Ng-Kwai-Hang et al., 1982). Serum albumin, immunoglobulins, transferrin and other serum proteins pass into the milk because of altered vascular permeability (Sheldrake et al., 1983; Reneau and Packard, 1991). Among other milk constituents, concentrations of sodium chloride, whey nitrogen increase, while that of potassium and lactose decrease with SCC exceeding 1.5 x 105 cells/ml milk (Reichmuth, 1975). A higher SCC value in milk is generally associated with greater lipase and aldolase activity, higher concentration of free and short chain fatty acids, higher acid degree values and lower xanthene oxidase activity (Randolph and Erwin, 1975).
Table 1: Variations in the milk quality of cow in relation to SCC [Alhussien et al., 2018].
| Milk constituents | Healthy | Subclinical mastitis | Clinical mastitis |
| SCC (105cells/ml) | <2 | 3-5 | >5 |
| Fat (%) | 4.32 | 4.31 | 4.08 |
| Protein (%) | 3.30 | 3.34 | 3.70 |
| Casein | 2.70 | 2.55 | 2.25 |
| Whey protein | 0.84 | 1.13 | 1.35 |
| Serum albumin | 0.17 | 0.24 | 0.37 |
| Lactose (%) | 4.84 | 4.71 | 4.41 |
| SNF (%) | 9.73 | 9.61 | 9.35 |
| pH | 6.61 | 6.63 | 6.80 |
| Chloride | 0.09 | 0.13 | 0.16 |
| Sodium | 0.05 | 0.09 | 0.11 |
Impact on public health
A higher SCC in milk associated with human health hazard. Pathogens like endotoxin producing Staplylococci spp. may be a problem even in pasteurized milk, since these endotoxins are thermostable (Tolle, 1975). Group B Streptococci (agalactiae) are pathogenic, but do not survive through pasteurization. Among other potential pathogens E. coli, Campylobacter jejuni, Yersinia enterocolitica, Listeria monocytogenes, Salmonella spp. and Clostridium spp. may cause food poisoning. Another concern on public health associated with higher SCC milk is increased use of antibiotics in order to lower SCC and clinical mastitis cases in the herd. Widespread use of antibiotics as prophylactic agent may cause antibiotic residues in food chain and subsequent natural selection for resistant strains (Jones, 1986). Microbial activity is required for acidity, flavour and curd characteristics of cultured dairy products. By inhibiting the microbial growth, antibiotics can alter the desirable characteristics of cultured dairy products (Oliver et al., 1984).
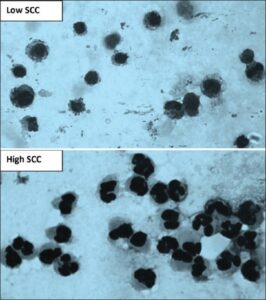
Fig 5: Microscopic examination of milk somatic cells smear showing that macrophages are the major cell in low somatic cell counts (SCCs) and neutrophils are the main cell in high SCC.
Impact on processors
With qualitative and quantitative changes of casein in higher SCC milk, the cheese yields are reduced. The quality of cheese deteriorates with flavour problem that worsen with the age of cheese (Richter, 1976). A decline in SCC from 2.9 x 105 to 2.0 x 105 cells/ml milk increased cheese yield by 4.5 percent (Leavitt et al., 1982). The alkaline pH of mastitic milk adversely affects rennet coagulation, decreased cure firmness (Everson, 1980). The higher SCC milk produces cream with longer churning time, butter with weakly body and 10 percent less milk fat globulin membrane (MFGM) that protect fat globules from lipolysis, photoactivation and oxidation (Randolph and Erwin, 1974). Shelf life of pasteurized dairy products may be influenced adversely along with flavour defect (Hoare et al., 1975). The heat stability properties of milk powder and quality of recombined evaporated and sweetened condensed milk also are deteriorated (Feagan et al., 1966) with high SCC.
Interpretation of SCC
Certainly the use of SCC records on fortnightly or monthly basis can be a useful tool in monitoring udder health in a dairy herd. Repeated SCC results on individual cows or at the farm level are most useful, whereas a single SCC test result can be relatively inconclusive. It should be stressed that interpretation of SCC records is particularly applicable to herds experiencing infections due to contagious pathogens. Since infections by these pathogens tend to be of long duration, new infections in the herd may lead to increased prevalence of infection and is reflected in elevated SCC in bulk tank or herd average SCC scores. Some researchers found relationship between average milk cell count, udder health and quality of milk. If quarter milk has 1 lakh/ml, the udder is considered healthy. If the count exceeds 2.5 lakhs/ml, there is presumed that there are some disturbances in secretary process. A cell count of 3 lakhs/ ml or above indicates the mammary gland has got infection, and cell count above 5 lakhs/ml means the udder is diseased. Well-managed herds that have controlled mastitis due to contagious pathogens can experience clinical mastitis problems due to environmental pathogens, yet maintain herd average SCC below 300,000 (Hoblet et al., 1991). In this case the long-term udder health status may not be clearly reflected in monthly herd SCC or BTSCC. Intramammary infections by environmental pathogens tend to be of shorter duration than those caused by contagious pathogens; 60 to 70% of these environmental infections may be less than 30 days in duration (Hogan and Smith, 1987). The time period of elevated SCC in these cows would be correspondingly shorter as well. The prevalence of infection by environmental pathogens at any time point also tends to be low (less than 10% of quarters in the herd). Thus, herds that have predominantly environmental mastitis may have BTSCC below 300,000 (some may be below 200,000); because the relatively small number of environmental infections in the herd at any particular time is not having a major impact on the herd SCC. Exceptions may be during times of increased clinical incidence, when there are greater than usual numbers of clinical cases at once.
Somatic cell counts in sheep and goat
Goats
The goat milk has higher SCC than bovine milk and usually it is about 1 x 106 cells per ml of normal milk. The cell populations mainly consist of polymorphonuclear leukocytes (50-70%) and their proportion to other cell type increases with the advancement of lactation. (Paape and Capuco, 1997; Rota et al., 1993). In a study, 50 percent of milk samples with SCC value of more than 106 cells/ml of milk were found negative on bacterial culture (White and Hinckley, 1999; Zeng and Escoban, 1996). Another study revealed that with mild disturbances in udder secretion the mean SCC level was 2.144 x 106 cells/ml milk (Kosev et al., 1993). Types of infection can considerably alter the pattern of elevation of SCC in milk. Infection with S. aureus usually results in sharp rise of SCC values ranging from 4-8 x 106 cells/ml milk (Lerondelle et al., 1989; Poutrel et al., 1997). Coagulase negative staphylococci infections are less predictable to cause elevation in SCC levels. Infection with some species such as S. chromogenes and S. hyicus can cause a rise of SCC level of 2 x 106 cell/ml milk, whereas S.epidermidis infection is associated with lower SCC level (1.4 x 106 cells/ml milk) (Sanchez et al., 1998). Threshold value of 0.5 x 106 cells/ml milk to determine IMI had a negative predictive value of 90 %. However, this threshold can be used as a basis for screening samples for bacteriological culture. One important point in counting SCC of goat milk is that counting methods that are DNA specific should be used. The milk secretion in goat is of apocrine type rather than marocrine (cattle) result into secretion of cytoplasmic particles in milk including a small portion of RNA fragment (Paape and Capuco, 1997). Direct microscopic counting with DNA specific strain like pyronin Y or methyl green should be used (Droke et al., 1993) where fossomatic electronic cell counter is not available. In the latter, the machine should be calibrated with goat milk rather than cow milk standards (Zeng, 1996).
Sheep
The association of SCC with intra mammary infection in sheep is rather obvious compared to goat and thus can be a potent tool to detect IMI. Threshold value of 1 x 106 cells/ml milk can be suitably used to distinct between healthy and infected sheep (Fthenakis, 1994; Fthenakis et al., 1991; Maisi et al., 1997). In normal milk macrophages make up the 50-70% of the cells with polymorphonuclear leucocytes (15- 40%), lymphocytes (6-14%) and other cell types (<5%). Stage of lactation is likely most important factor to alter SCC level aside from infection with higher mean SCC level towards the end of lactation (Fthenakis, 1996). Other factors, which may affect SCC levels, are lactation number (Rota et al., 1993; Zeng, 1996), time of the day with higher values in morning milk samples (Fthenakis, 1994) and management (Ubertalle et al., 1993).
Use of SCC in breeding for mastitis resistance
SCC is mostly used trait employed by different countries in their selection programme to improve mastitis resistance. It has high heritability (0.08 to 0.19) than mastitis (Luttinen and Juga, 1997) and has high genetic correlation (average 0.7) with mastitis (Coffey et al., 1986). It is easy to record regularly and an indicator of clinical and sub clinical mastitis. Selection for lower SCC gave 93% of the response of that obtained through direct selection of mastitis data and is more efficient than direct selection when progeny group size is small. The drawbacks of using SCC in breeding for mastitis resistance are that it may impair the animal’s innate immune systems and reduce its ability to respond against udder pathogens (Schukken et al., 1994). SCC as a predictor of mastitis is dependent on the level of mastitis in the herd and is more efficient when incidence of mastitis is high. Sometimes increase in SCC during mastitis caused by E. coli is of short duration and may not be detected by monthly sampling of SCC.
Conclusion
Milk somatic cell count is the indicator for the milk quality and udder health.This review highlights the requirements and importance of the Milk SCC and it’s effects in public health.The subclinical mastitis is the main problem which leads to huge economic loss to farmers and country and also it reduces the shelf life of milk and milk products.Further we should consider the milk SCC as the main parameter as the indicator of managemental quality and animal welfare.
Acknowledgments
The authors thank every researchers for their outstanding contribution to several of the research studies presented in this review. The authors are grateful to Manisha, Wonchi,Roohani,Gagan, Aneesh, Akanksha, Yamini, Akhil, Simran, Amandeep, Suvarna, Swathi, Indresh, Asif ali for their careful editing of this manuscript.
Bibliography
Alhussien,M.N. Dang, A.K. 2018 . Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet World.11(5): 562–577.
Andrews, R. J., Kitchen, B. J., Kwee, W. S. and Duncalfe, F. 1983. Relationship between individual cow somatic cell counts and mastitis infection status of the udder. Aust. J. Dairy Tevhnol. 38:71.
Asby, C. B., Gard, R. P. and Watkins, J. H. 1977. The relationship between herd bulk milk composition and cell count in commercial dairy herds. J. Dairy Res. 44: 585.
Burvenich, C., Guidry, A. J. and Paape, M. J. 1995. Natural defence mechanisms of the lactating and dry mammary gland. In Proc.3rd Int. Mastitis Sem., Tel Aviv, Israel, S1, 3-13.
Coffey, E.M., Vinston, W.E. and Pearson, R.E. 1986. Potential use of somatic cell concentration in milk as a sire selection criteria to reduce mastitis in dairy cattle. J. Dairy Sci., 69: 2163-2172.
Dohoo, I.R. and Meek, A. H. 1982. Somatic cell counts in bovine milk. Can. Vet. J. 23:119.
Droke, E. A., Paape, M. J. and Di Carlo, A. L. 1993. Prevalence of high somatic cell counts in bulk tank goat milk. J. Dairy Sci. 76: 1035-1039.
Dulin, A. M., Paape, M. J., Schultz W. D. and Weinland, B. T. 1983. Effect of parity, stage of lactation and intramammary infection on concentration of somatic cells and cytoplasmic particles in goat milk. J. Dairy Sci. 66:2426-33.
Duris, G.F. 1978. Milking management and mastitis in Sri Lanka. New Zealand J. Agri., 136: 2224-2228.
Eberhart, R.J., Gilmore, H.C., Hutchinson, L. J. and Spencer, S.B. 1979. Somatic cell counts in DHI samples. Proc. Ann. Mtg. Natl. Mastitis Counc., p. 32.
Eberhart, R.J., Hutchinson, L. J. and Spencer, S.B. 1982. Relationships of bulk tank somatic cell counts to prevalence of intramammary infection and to indices of herd production. J. Food Protect. 45:1125.
Eberhart, R.J., Hutchinson, L.J. and Spencer, S.B. 1982. Relationships of bulk tank somatic cell counts to prevalence of intramammary infection and to indices of herd production. J. Food Prot., 45: 1125-1128.
Elvinger, F., Hansen, P.J. and Natzke. R.P. 1991. Modulation of function of bovine polymorphonuclear leukocytes and lymphocytes by high temperature in vitro and in vivo. Am. J. Vet. Res. 52: 1692.
Elvinger, F., P.J. Hansen, and R.P. Natzke. 1991. Modulation of function of bovine polymorphonuclear leukocytes and lymphocytes by high temperature in vitro and in vivo. Am. J. Vet. Res., 52: 1692.
Everson, T. C. 1980. How the dairy industry can benefit from a somatic cell programme. In: Proc. 19th Annu. Mtg. Natl. Mastitis Counc. Arlington, VA. pp: 153.
Fthenakis, G. C. 1994. Prevalence and aetiology of subclinical mastitis in ewes of southern Greece. Small Ruminant Res. 13: 293-300.
Fthenakis, G. C. 1996. Somatic cell counts in milk of Welsh Mountain, Dorset-Horn and Chios ewes throughout the lactation. Small Ruminant Res. 20: 155-162.
Fthenakis, G. C., El-Masannat, E. T. S. and Booth, J. M. 1991. Somatic cell counts of ewe’s milk. British Vet. J. 147: 575-581.
Ghosh ,C.P., Nagpaul, P.K. and Roy, B. 2001. Studies on impact of milking systems on udder health. Indian J. Anim. Prod. & Mgmt.
Ghosh,C.P., Nagpaul, P.K. and Shiv Prasad. 2004. Prevalence of Subclinical Mastitis in Cattle and Buffaloes and its Impact on Somatic Cell Counts and Various Milk Constituents. Indian J. Dairy Science., 57: 329-333.
Ghosh, C.P and Prasad. S. 2007. Effect of two different cooling strategies on microclimate, physiological reactions and production performance of crss bred cows during summer. Ind J Animal Sci., 77 (12): 1238-1243.
Haenlein, G. F. W., Schultz, L. H. and Zikakis, J. P. 1973. Composition of proteins in milk with varying leukocyte contents. J. Dairy Sci. 56: 1017.
Hamann, J. and Stanitzke, U. 1991. Comparison of reactions of the teat tissue to calf suckling, hand milking and machine milking with special emphasis on pathogenesis of bovine mastitis. Brief Communications of the 23rd International Dairy Congress, Montreal, October 8-12, 1990, Vol.1, p. 270. Brussels, Belgium: International Dairy Federation.
Hoare, R. J. T., Dettmann, B. and benson, R. J. 1975. The New South Wales mastitis control programme. In: Proc. IDF Seminar on Mastitis Control. Int. Dairy Fed. Brussels. pp-491.
Hoblet, K.H., Schnitkey, G.D., Arbaugh, D., Hogan, J.S., Smith, K.L., Schoenberger, P.S., Todhunter, D.A., Hueston, W.D., Pritchard, D.E., Bowman, G.L., Heider, L.E., Brockett, B.L. and Conrad. H.R. 1991. Costs associated with selected practices and with episodes of clinical mastitis in nine herds with low somatic cell counts. J. Am. Vet. Med. Assoc. 199: 190.
Hogan, J.S. and Smith, K.L. 1987. A practical look at environmental mastitis. Compendium on Continuing Education for the Practicing Veterinarian. 9: F341.
Hogan, J.S., Smith, K.L., Hoblet, K.H., Todhunter, D.A., Schoenberger, P.S., Hueston, W.D., Pritchard, D.E., Bowman, G.L., Hueston, W.D., Heider, L.E., Brockett, B.L. and Conard, H.R. 1989. Bacterial counts in bedding materials used on nine commercial dairies. J. Dairy Sci., 72: 250-258.
International Dairy Federation Bulletin. 1984. Recommendation methods for somatic cell counts in milk. I.D.F. No. 168, pp. 4- 6.
Kirk, J. H., Berry, S. L., Reynolds, J. P., Maas, J. P. and Ahmadi, A. 1996. Sensitivity and specificity analysis for somatic cell count (SCC) used to predict bacteriologically positive subclinical mastitis at calving in a dairy herd with low SCC. J. Amer. Vet. Med. Assoc. 208: 1054-57.
Kosev, K., Tzolov, S. and Denev, S. 1993. influence of the different forms of udder inflammation and the species of microbial agents on the somatic cell counts in goats’ milk. In: Proceedings of the Sixth International Symposium on Somatic Cells and Milk of Small Ruminants. Bella, Italy. pp: 107-109.
Laevens, H., Deluyker, H., Schukken, Y.H., de Meulemeester, L., Vandermeersch, R., de Muelenaere, E. and de Kruif, A. 1997. Influence of parity and stage of lactation on somatic cell count in bacteriologically negative dairy cows. J. Dairy Sci. 80:3219.
Lee, C.S., Wooding, F.B.P. and Kemp, P. 1980. Identification properties, and differential counts of cell populations using electron microscopy of dry cows secretions, colostrum and milk from normal cows. J. Dairy Res. 47:39.
Lerondelle, C., Richard, Y. and Issartial, J. 1992. Factors affecting somatic cell counts in goat milk. Small Ruminant Res. 8: 129- 139.
Lin, C. J. and Chang, H. S. 1994. Studies on relationship between somatic cell counts and milk quality in goat. J. Chinesse Soc. Anim. Sci. 23: 407-18
Lutinnen, A. and Juga, J. 1997. Genetic relationship between milk yield, somatic cell count, mastitis, milkability and leakage in Finish dairy cattle. Inter. Bull Bulletin no.15, pp. 77-83. Maisi, P., Juntilla, J. and Seppanen, J. 1987. Detection of subclinical mastitis in ewes. British Vet. J. 143: 402-409.
Miller, R. H. and Paape, M. J. 1985. Relationship between milk somatic cell count and milk yield. In: Proc. 24th Annu. Mtg. Natl. Mastitis Counc. Arlington, VA. pp: 60.
Miller, R.H. and Paape, M.J. 1985. Relationship between milk somatic cell count and milk yield. Proc. Ann. Mtg. Natl. Mastitis Counc., p. 60. Muggali, J. 1995. Influence of somatic cell counts on stage of lactation. Anim. Breed. Abstr. 1996.
Nelson, F.E., Tranmal, H., Schuh, J.D., Wegner, T.N. and Scott, G.H. 1969. Criteria of abnormal milk from individual quarters during period of high temperature. J.Dairy Sci., 52: 912.
Ng-Kwai-Hang, K. F., Haynes, J. F., Moxley, J. E. and Monardes, H. G. 1982. Environmental influences on protein content and composition of bovine milk. J. Dairy Sci. 65: 1993.
Paape, M. J. and Capuco, A. V. 1997. Cellular defence mechanisms in the udder and lactation of goats. J. Anim. Sci. 75: 556-565.
Paape, M. J. and Contreras, A. 1997. Historical perspective on the evolution of milk somatic cell count. Flem. Vet. J. Suppl., 62- 95.
Philpot, W. N. 1978. Mastitis management. Babson Bros. Co., Oak Brook, IL. Randolph, H. E. and Erwin, R. E. 1974. Influence of mastitis on properties of milk. X. Fatty acid composition. J. Dairy Sci. 57: 865.
Randy, H. A., Caleer, W. A. and Miner, W. H. 1991. Effect of lactation number, year and milking management practices on milk yield and SCC of French Alpine dairy goats. J. Dairy Sci. 74(suppl. 1): 278
Reichmuth, J. 1975. somatic cell counting – interpretation of results. In: Proc. IDF Seminar on Mastitis Control, Int. Dairy Fed., Brussels. pp: 93.
Reneau, J.K. 1986. Effective use of Dairy Herd Improvement somatic cell counts in mastitis control. J. Dairy Sci. 69:1708.
Reneau, J.K. and Packard, V.S. 1991. Monitoring mastitis, milk quality and economic losses in dairy fields. Dairy Food Environ. Sanitation 11:4.
Richter, R. L. 1976. the effect of mastitis on the processing properties of milk. In: Proc. 15th Annu. Mtg. Natl. Mastitis Counc. Arlington, VA. pp-25.
Rota, A. M., Gonzalo, C. and Rodriguez, P. L. 1993. Somatic cell types in goats’ milk in relation to total cell count, stage and number of lactation. Small Ruminant Res. 12: 89-98.
Roussel, J.D., Lee, J.A., Beatty, J.F. and Gholson, J.H. 1969. Effect of thermal stress on somatic cell counts and milk constituents and blood cells. J. Dairy Sci., 52: 562-567.
Sanchez, A., Corrales, J. C. and Luengo, C. 1998. Intramammary pathogens and somatic cell counts in dairy goats. In: Proceedings of the Sixth International Symposium on Milking of Small Ruminants-Milking and Milk Production of Dairy Sheep and Goats. Athens, Greece. pp: 124-129.
Sargeant, J. M., Leslie, K. E., Shirley, J. E., Pulkrabek, B. J. and Lim, G. H. 2001. Sensitivity and Specificity of somatic cell count and California mastitis test for identifying intramammary infection in early lactation. J. Dairy Sci. 84: 2018-24.
Schalm, O.W., Carou, E.J. and Jain, N.C. 1971. Bovine mastitis. J. Amer. Vet. Med. Assoc., 150: 33-37. Schukken, Y.H., Mallard, B.A., Dekkers, J.C.M., Leslie, K.E. and Stear, M.J. (1994). Genetic impact on the intramammary infection following Staphylococcus aureus challenge. J.Dairy.Sci.77:639-647.
Sears, P.M.,. Smith, B.S., English, P.B., Herer, P.S. and Gonzalez, R.N. 1990. Shedding pattern of Staphylococcus aureus from bovine intramammary infections. J. Dairy Sci. 73: 2785.
Shearer, J.K. and Beede, D.K. 1990. Effects of high environmental temperature on production, reproduction, and health of dairy cattle. Agri. Pract. 11: 6.
Sheldrake, R.F., Hoare, R. J. T. and McGregor, G. D. 1983. Lactation stage, parity and infection affecting somatic cells, electrical conductivity, and serum albumin in milk. J. Dairy Sci. 66:542
Silva, L.D. and Silva, K.F.S.T. 1994. Total and differential cell counts in buffalo (bubalus bubalis) milk. Buffalo Journal, 2: 133-137.
Singh, M. 2002. Somatic cell counts during lactation in bovines as an index of subclinical mastitis. All India Dairy Husbandry Officers Workshop. pp. 64-76
Singh, M. and Ludri, R. S. 2001. Influence of stages of lactation, parity and season on somatic cell counts in cows. Asian-Aust. J. Anim. Sci. 14: 1775-80.
Singh, Mahendra and Ludri, R.S. 2002. Somatic cell counts in Murrah buffaloes during different stages of lactation, parity and season. Asian-Aus J. Anim. Sci., 14(2): 189-192.
Tolle, A. 1975. Mastitis-the disease in relation to control methods. In: Proc. IDF Seminar on Mastitis Control. Int. Dairy Fed., Brussels. pp-3.
Ubertalle, A., Battaglini, L. M. and Fortina, R. 1993. Effect of some variation factors on somatic cell counts in Delle Langhe sheep milk. In Proceedings of the Sixth International Symposium on Somatic Cells and Milk of Small Ruminants. Bella, Italy. pp: 187-192.
Wegner, T.N., Schuh, J.D. Nelson, F.E. and G.H. Stott. 1976. Effect of stress on blood leukocyte and milk somatic cell counts in dairy cows. J. Dairy Sci., 59: 949.
White, E. C. and Hinckley L. S. 1999. Prevalence of mastitis pathogens in goat milk. Small Ruminant Res. 33: 117-121.
Whittleston, W.G.F., Kilgour, R., Langer, De H. and Duris, G. 1970. Behavioural stress and somatic cell count of bovine milk. J. Food Technol., 33: 271-280.
Zeng, S. S. 1996. Comparison of goat milk standards with cow milk standards for analyses of somatic cell count, fat and protein in goat milk. Small Ruminant Res. 21: 221-225.
Zeng, S. S. and Escobar, E. N. 1996. Effect of breed and milking method on somatic cell count and composition of goat milk. Small Ruminant Res. 19: 169-175.