SOMATIC CELL – AN INDICATOR OF UDDER HEALTH & MILK QUALITY IN DAIRY CATTLE
by-DR RAJESH KUMAR SINGH ,JAMSHEDPUR,JHARKHAND, INDIA, 9431309542,rajeshsinghvet@gmail.com
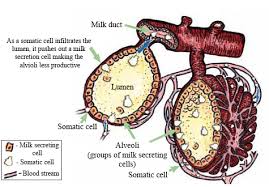
The somatic cell count (SCC) is a main indicator of milk quality. The majority of somatic cells are leukocytes (white blood cells) – which become present in increasing numbers in milk usually as an immune response to a mastitis-causing pathogen – and a small number of epithelial cells (milk producing cells) which are shed from the inside of the udder as a result of infection.
SCC is quantified as the number of cells per ml of milk. In general terms:
•
An individual cow SCC of 100,000 or less indicates an uninfected cow, where there are no significant production losses due to subclinical mastitis.
• A threshold SCC of 200,000 would determine whether a cow is infected with mastitis.
Cows with an SCC of greater than 200,000 are highly likely to be infected in at least one quarter.
• Cows infected with significant pathogens have an SCC of 300,000 or greater.
The SCC in milk increases after calving when colostrum is produced before the cow settles into lactation and tends to rise towards the end of lactation, most likely due to lower volumes of milk being produced and so a concentrating effect may come into play. SCCs vary due to many factors, including seasonal and management effects.
Dairy farmers are financially rewarded for having low SCCs and penalised for high ones. Milk with a value of 400,000 or above is deemed to be unfit for human consumption.
WHAT ARE SOMATIC CELLS?—
Somatic cells are mainly milk-secreting epithelial cells that have been shed from the lining of the gland and white blood cells (leukocytes) that have entered the mammary gland in response to injury or infection (Dairyman’s digest, 2009). The milk somatic cells include 75% leucocytes, i.e. neutrophils, macrophages, lymphocytes, erythrocytes, and 25% epithelial cells. Erythrocytes can be found at concentrations ranging from 0 to 1.51×106 /ml (Paape and Weinland, 1988). Studies identifying cell types in milk have shown that epithelial cells or the cells which produce milk are infrequently found in udder secretions, including the dry gland, at levels ranging from 0 to 7% of the cell population (Lee et al., 1980). The epithelial cells of the glands are normally shed and get renewed, however, during infection the numbers increase. The white blood cells serve as a defense mechanism to fight infection and assist in the repair of damaged tissue. During inflammation (mastitis) the major increase in SCC is due to the influx of neutrophils into the milk to fight infection and have been estimated at over 90% (Miller and Paape, 1985; Harmon, 1994) and the measurement of SCC in milk is known as a somatic cell count. The normal composition of milk somatic cells varies with the type of secretion or lactation cycle (Table 1). Normally, in milk from a healthy mammary gland, the SCC is lower than 1×105 cells/ml, while bacterial infection can cause it to increase to above 1×106 cells/ml (Bytyqi et al., 2010).
FUNCTION OF SOMATIC CELLS—–
Mastitis is caused by bacterial invasion into the udder. The small numbers of somatic cells that are normally present in milk attempt to resolve this intramammary infection immediately. The cellular presence in milk is one of the important protective mechanisms of the mammary gland and may be considered as a surveillance function in the uninfected gland. Both bacteria and leukocytes in the infected quarters release chemo-attractive products for leukocytes, especially neutrophils. The neutrophil polymorphonuclear (PMN) leukocytes are the second line of defense against mammary gland infection. PMN’s are phagocytic cells which engulf and kill bacteria. However, in bovines, the phagocytic ability of PMN of milk can consume milk fat globules and casein (Opdebeeck, 1982) leading to putrefaction of milk. An inflammatory response is usually initiated when bacteria enter the mammary gland through the teat canal and multiply in the milk. Although bacterial toxins, enzymes and cell-wall components have a direct effect on the function of the mammary epithelium, they it also stimulate the production of numerous mediators of inflammation, mainly neutrophils (Gallin et al., 1992), due to edema, vasodilation and increased vascular permeability Blood monocytes become macrophages in the tissues and are the major cell type in milk during involution of the udder. During bacterial pathogenesis, macrophages serve to facilitate either innate or acquired immune responses. During lactation, the proportion of macrophages is highest (68%) in the early post-partum period and lowest (21%) in late lactation (Park et al., 1992). Similar to neutrophils, the non-specific functions of macrophages are to phagocytise invading bacteria and destroy them with proteases and reactive oxygen species (ROS) (Mullan et al., 1985). Lymphocytes are the only cells of the immune system that recognize a variety of antigenic structures through membrane receptors, which define their specificity, diversity and memory characters (Boyso et al., 2007). T-lymphocytes and B-lymphocytes are two subsets of lymphocytes that differ in function and protein products and play specific immune functions (Harmon, 2001). The mammary epithelial cells may play a protective role in prevention of infection via ingestion and possible digestion of phagocytosed microbes. The mammary epithelial cells are able to produce a variety of inflammatory mediators such as cytokine, chemokines, host defense peptides and arachidonic acid metabolites.
FACTORS AFFECTING SOMATIC CELL COUNT——-
There are plenty of factors that influence milk somatic cell count at individual and herd level apart from intramammary infection. The ability to correctly interpret somatic cell counts depends on an understanding of the factors which may affect the number of somatic cells.
Mammary gland infection level (Mastitis)
The most important factor affecting the somatic cell count of the milk from an individual quarter depends upon the infection status of the quarter (Dohoo and Meek, 1982). Sharma (2003) analyzed 2161 milk samples from lactating cows and demonstrated that SCC ≤100,000 cells/ml could be considered as threshold or negative for the California mastitis test (CMT) (Figure 1). The degree and nature of the cellular response are likely to be proportional to the severity of the infection (Figure 2). The average number of composite (cow) milk SCC increases with an increase in the number of quarters infected (Meek et al., 1980) and having a major influx of PMN into the milk (Craven and Williams, 1985; Miller et al., 1990).
Stage of lactation
SCC increases with progressing lactation (late lactation) regardless of whether the cow is infected or not (Dohoo and Meek, 1982). SCC elevation has been linked with an animal’s innate immune response in preparation for calving and to enhance the mammary gland defense mechanism at this critical calving time (Reichmuth, 1975). During early and late lactation the percentage of neutrophils tends to increase while the percentage of lymphocytes decreases (McDonald and Anderson, 1981). At parturition SCC are usually higher than one million per ml and decreases to 100,000 cells/ml in the 7 to 10 days post-partum (Jensen and Eberhart, 1981) (Table 2). The presence of high cell numbers has also been reported in colostrum and appears due to an excessive desquamation of epithelial cells in a small volume of milk in a gland resuming functional activity after a dormant period
Age/Breed—-
Various researchers have reported that SCC increases with increasing age (Beckley and Johnson, 1966; Blackburn, 1966) (Table 3). This increase is primarily due to an increased prevalence of infection in older cows and is not due to any large increase due to age per se (Reichmuth, 1975). SCC variation has been noted between breeds of dairy animals. The high-producing cattle breeds such as Brown Swiss (423.31×103 cells/ml) and Black Holstein (310.36× 103 cells/ml) have higher presence of SCC/ml in milk. Different Indian breeds of cows with their SCC’s have been depicted by Singh (2002) and are shown in Table 4.
Parity/Season/Stress—–
The level of SCC has been reported to be influenced by parity (Blackburn, 1966; Lindström et al., 1981). There is little change in SCC of uninfected quarters as number of lactations increases (Sheldrake et al., 1983) but SCC increases with advanced parities (Skrzypek et al., 2004). Somatic cell counts are generally lowest during the winter and highest during the summer season (Khate and Yadav, 2010). During summer, the growth and number of environmental bacteria is increased in the bedding material of housed stock due to favorable temperature and humidity (Harmon, 1994). Free radicals are generally produced during stress due to milking techniques, environmental and infectious organisms (teat injury). These radicals are unstable and react quickly with other compounds in order to capture the electron to gain stability
Diurnal variation——
In general, SCC that is lowest just before milking increases rapidly on stripping, and may persist for up to 4 hours after milking and then gradually declines. This difference in high and low SCC varies from 4 to 70-fold for individual quarters (White and Rattray, 1965). Studies have also shown that two consecutive milkings from the same cow could fluctuate in SCC by 30%. Day to day variation in cell counts has also been investigated and revealed that SCC could fluctuate to more than 40% without any of the circumstances described above
Milk transportation/Management——
Methods of transportation and storage of milk samples have been demonstrated to affect SCC count (Dohoo et al., 1984). Gonzalo et al. (2003) used different milk preservatives e.g. potassium dichromate (100 mg/100 ml), azidiol (24 mg sodium azide/100 ml) and bronopol (50 mg/100 ml) for counting and revealed the highest SCC in samples without preservative (5.72×103 cells/ml), with bronopol (5.67×103 cells/ml), potassium dichromat(5.63×103 cells/ml) and azidiol (5.62×103 cells/ml). There are many management factors that play a most important role in the development of contagious disease like mastitis in dairy animals. Amongst these, unhygienic conditions are more important in increasing the chances of intramammary infection (IMI) and resulting in high SCC. Other management factors pertain to the type of flooring, feeding, teat dipping and milking techniques etc. Teat injuries and leakers commonly develop because of stall and platform design raising the incidence of mastitis and causing higher SCC. Using a post-milking teat dip appears to predispose some very low SCC herds to more clinical mastitis-in particular mastitis caused by E. coli. Recently, hygienic milking has come into practice routinely to prevent the spread of Staph. aureus inflicting contagious mastitis.
METHOD FOR MEASURING SOMATIC CELLS—–
More recently, automated devices for rapidly determining the SCC of milk samples have become available. On-going development in counting technology has resulted in the routine application of high capacity flow cytometric counters with much improved performance in advanced milk testing laboratories. The two most commonly-used devices are the Coulter Milk Cell Counter, which counts particles as they flow through an electric field, and the Fossomatic which stains cells with a fluorescent dye and then counts the number of fluorescing particles. Both devices are capable of rapid determination of the SCC in large numbers of samples. Details of the procedures used by each device have been published by various workers (Heeschen, 1975; Gonzalo et al., 2003) and will not be discussed further in this paper. The direct microscopic method is inexpensive and most commonly used in India (Sharma, 2003) (Figure 3). However, there is very little information on the specific application of these methods in ewe milk (Gonzalo et al., 1993), which has a higher content of total solids than cow milk. When evaluating macrophages on a stained milk film, many will have a “foamy” cytoplasm that could be analysed using the Fossomatic method (Gonzalo et al., 2003).
SCC BASED INTERPRETATIONS—–
The two different methods may be used to calculate an “average somatic cell count” when multiple samples have been taken. For example, if the past three-month cell counts were 600,000, 400,000 and 500,000, the average would be calculated by arriving at a total and dividing by 3, (1,500,000/3=500,000). This produces an “arithmetic mean” or average. A different method, used in Europe and other locations, is used to calculate an average somatic cell count. It is termed the “geometric mean”. The geometric mean calculation always produces a value somewhat less than the arithmetic mean for the same data set. A single high count in a data set has a greater impact on the arithmetic mean than the geometric mean and one very high value is not as likely to trigger regulatory action using the geometric mean procedure (Ingalls, 2001). Research has established a straight-line relationship between milk loss and the logarithm of the SCC. This value is referred to in Canada as “Linear Score” (LS) and in the US as Somatic Cell Score (SCS). Increase in linear score with the doubling of SCC has been recorded by Ingalls (2001) as shown in Table 5. All lactating cows have a low baseline SCC even if they do not have an intramammary infection (IMI). When an infection is detected by the immune system in a healthy cow, a rapid influx of leukocytes will quickly raise the SCC far beyond the baseline level, usually to over a million cells/ml. In most developed dairy industries various regulatory limits has been applied to milk for human consumption. The European Union Directives (92/46CEE and 94/71 CEE) set a limit of 400,000 cells/ml for SCC in raw buffalo milk
when the milk is used for products made with raw milk. In US, the legal maximum somatic cell count for Grade A farm bulk milk is 750,000 cells/ml, this limit is high compared to many international standards. Much of Europe, New Zealand and Australia has a limit of 400,000 cells/ml and Canada has a limit of 500,000 cells/ml of raw milk. Milk SCC is a diagnostic figure for subclinical mastitis (International Dairy Federation, 1999). Cow milk SCC of >200,000 cells/ml indicates mastitis (International Dairy Federation, 1997; Hillerton, 1999). Recently, a line has been drawn for SCC that a level below 100,000 cells/ml represents a healthy quarter. However, some researchers consider a normal SCC to be up to 500,000 cells/ml. However, it has been proposed that quarters having a cell count of 200000 cells/ml and whole cow milk cell count of 400,000 cells/ml to indicate mastitis (Hillerton, 1999). Therefore, mastitis should be detected in a reliable and timely fashion based on SCC values, otherwise subclinical mastitis could develop into a clinical disease (Hallén Sandgren et al., 2008).
SCC AND MASTITIS CAUSING ORGANISM——
High SCC present in milk is the main indicator of mammary gland infection, caused by specific and nonspecific micro-organisms, which cause contagious and environmental mastitis. The most common organisms that cause mastitis are classified into two major groups: i) contagious pathogens and, ii) environmental pathogens. The contagious pathogens (Staphylococcus aureus, Streptococcus agalactiae) generally cause the greatest SCC increase. An infection by environmental pathogens (Strep. dysgalactiae, Strep. uberis, Corynebacterium bovis and Coagulase negative Staphylococcus) usually causes considerably less SCC elevation.
SCC increases of greater than 200,000 cells/ml have been observed in cow milk as a result of bacterial infection. Various major or minor pathogens display a moderate increase in somatic cells of approximately 50,000 cells/ml. The magnitude of SCC response to major pathogens varies among cows, however, differentiation of types of pathogens seem impossible based on SCC alone (Dohoo and Meek, 1982). A study conducted by Boddie et al. (1987) showed the mean SCC of quarters from unbred heifers infected with Staph. chromogenes, Staph. hyicus, and Staph. aureus were 7.8, 8.5, and 9.2×106 cells/ml, respectively. The mean SCC of uninfected quarters was 3.5×106 cells/ml. The mean SCC of heifer secretions collected on the day of freshening were 3.2×106 and 1.6×106 cells/ml for quarters infected by staphylococci and uninfected quarters, respectively. The mean SCC during the first 3 months of lactation in quarters infected with Staph. chromogenes, Staph. hyicus, and Staph. aureus were 168, 193, and 578×103 cells/ml, respectively, and SCC of uninfected quarters was 39×103 cells/ml. However, SCC approached 20×106 cells/ml in quarters infected with Staph. aureus and over 13.6×106 cells/ml in those infected with coagulase-negative staphylococci (CNS) and Streptococcus species. Such elevated SCC over a long period of time suggests that affected quarters were in a state of chronic inflammation, which could adversely affect development of milk-producing tissues (Nickerson, 2009). Sheldrake et al. (1983) compared lactation curves for SCC of quarters free from clinical mastitis with lactation curves for SCC of quarters with clinical Staph. aureus, coagulase-negative staphylococci (CNS), and Corynebacterium bovis mastitis. He revealed that quarters with clinical Staph. aureus mastitis showed a considerable increase in SCC and quarters with known infection had higher SCC than quarters
free from clinical mastitis. Schepers et al. (1997) showed how different pathogens caused changes or increases in quarter SCC. The largest increase was found for Staph. aureus and the smallest for Corynebacterium bovis. Malinowski et al. (2006) carried out a study to determine the relationship between SCC and mastitis etiological agents. They reported that milk samples with SCC lower than 200,000 cells/ml were mostly (59.6%) culture negative. Coagulase-negative staphylococci (CNS), Staph. aureus and Streptococcus sp. were mostly noted in samples with 200,000 to 2,000,000 of SCC/ml. Samples having more than 2 million/ml of SCC were infected mainly with CAMP-negative and CAMP-positive streptococci and Gram negative bacilli. The highest SCC (≥10 million/ml) in foremilk samples were associated with intramammary infections by Arcanobacterium pyogenes (95.5%), Streptococcus agalactiae (57.6%) and Gram-negative organisms (46.5%). Very high SCC (≥5 million/ml) was connected with infections caused by Prototheca sp. (64.5%), yeast-like fungi (60.2%) and Streptococcus sp. (55.1%). Staph. aureus (76.2%), CNS (84.2%), Gram-positive bacilli (72.4%) and Corynebacterium sp. (83.2%) caused an increase in SCC that was smaller than 5 million/ml.
EFFECT OF SCC ON MILK QUALITY AND HUMAN HEALTH————–
Subclinical mastitis alters the composition of the milk in addition to suppressing milk yield (Bramley, 1992; Harmon, 1994). Unlike milk production loss, there is a direct relationship between SCC and milk quality (Table 6). An elevated SCC in milk has a negative influence on the quality of raw milk. Subclinical mastitis is always related to low milk production (Bramley, 1992; Harmon, 1994), changes to milk consistency (density), reduced possibility of adequate milk processing, low protein and high risk for milk hygiene since it may even contain pathogenic organisms. The relationship between dairy cattle health and human health warrants mention. The dairy industry strives to produce milk and dairy foods that are safe and nutritious, and that are seen to be healthful and wholesome. The greatest risk of high SCC milk to human health is in the consumption of unpasteurized or improperly pasteurized milk (Oliver et al., 2005). Viable pathogens and their toxins can be transferred from the milk of infected quarters directly to humans. A large and diverse group of human pathogens reside in the cow’s environment including Salmonella dublin, Campylobacter jejuni, and Listeria monocytogenes (Oliver et al., 2005). These microbes are often pathogens or normal flora of dairy cows. Evidence has been reported that Mycobacterium avium subsp. paratuberculosis, associated with Johnes in cattle and isolated from human patients with Crohn’s disease, may survive some accepted milk pasteurization procedures. Although the possible association between shedding of the Mycobacterium avium subsp. paratuberculosis in milk and subsequent survival after pasteurization is compelling, the rate of shedding is low in infected cows and not related to an increase in SCC (Stabel, 2005). Surveys indicate that dairy producers and their families drinking milk produced on their own farms are among the demographic groups at greatest risk to food-borne diseases due to consumption of unpasteurized milk. Proper pasteurization of milk is very effective in preventing the transfer of viable pathogens from milk of infected mammary glands to humans. Pasteurization reduces the number of viable microorganisms, but often does not negate the effects of toxins produced by mastitis pathogens. There are a number of diseases of dairy cattle and pathogens transmissible from dairy cattle that are zoonotic. Direct ingestion of bovine neutrophils has been reported to cause health problems. As the SCC increases, the percentage of cells, particularly neutrophils, increases. Therefore, the potential health risk of consuming milk with an elevated SCC would depend largely on the human health concerns of ingesting bovine neutrophils. Although the ingestion of large numbers of bovine neutrophils in milk may be objectionable, direct negative effects on the safety of humans have not been documented as a result of consuming dairy products made with milk having high SCC.
REDUCTION AND MANAGEMENT OF HIGH SCC—————–
Bacterial invasion occurs mostly during the dry period, particularly during late gestation, and leads to glandular damage in parenchymatous tissue. The glandular tissue damage leads to increased SCC and reduced milk production. To reduce the occurrence of mastitis and control SCC, prevention strategies should be followed during the dry period. A few of the latest prevention strategies that have been recommended, which cover animal and environment hygiene, have included the use of teat sealants, teat antiseptics, pre-calving milking, control of insects and segregation of pregnant heifers from older cows etc. Furthermore, the standard mastitis control program decreases the prevalence of intramammary infections with contagious pathogens (Hillerton et al., 1995), but it has been rated relatively low in success in prevention of clinical mastitis from environmental pathogens (Lam et al., 1997b; Barkema et al., 1999). Recommendations to control both contagious and environmental pathogens have been combined in a new ten-point mastitis control program, issued by the National Mastitis Council (2001). In this program, lactation-average somatic cell count (SCC) has been generally used to control mastitis. The currently used primary parameters to analyse the herd situation in the mastitis control program are: i) bulk milk somatic cell count, ii) percentage of cows with SCC >250,000 cells/ml per test-day, iii) percentage of cows with new infections and iv) culling rate because of mastitis. Nutritional supplementation with vitamins and minerals enhances the immunity of the animal and therefore
decreases SCC numbers. Impact of such supplements have been already demonstrated with the use of vitamin E (at 500 IU/animal/d) and selenium (at 6 mg/animal/d) alone or in combination for two months during early lactation to control the intramammary infection and to manage SCC (Sharma and Maiti, 2005) (Figure 4). Such dietary supplementation of vitamin E and selenium in combination showed reduction in the SCC from 29.39×105 to 8.28×105 cells/ml of milk. Pre-calving antibiotic treatment was also found by Bastan et al. (2010) to be quite effective in reducing individual quarter SCC. Sharma et al. (2007) and Sharma (2008) have also reported a drastic decrease in SCC during clinical mastitis after treatment with enrofloxacin antibiotic Several studies in the past have shown a positive, unfavorable genetic correlation between milk yield and clinical mastitis (Shook, 1993; Rogers et al., 1998); this implies that genetic improvement for milk yield has been accompanied by increased genetic susceptibility to mastitis. Therefore, it is important to place some selection emphasis on udder health traits to offset the undesirable genetic trend towards mastitis susceptibility that results from selection for increased milk yield. Furthermore, there is also an utmost need for establishment of selection of an animal’s treatment for mastitis based on hematopoietic stem cell differentiation into innate immune cells and control of stem cell differentiation under the animal disease environment for discovery of a self-cure mechanism.