The Development Of Lumpy Skin Condition In India
Bhand Akshata Chandrakant1, Deshmukh Kaivalya Ruprao2, G Yashaswini3
1PhD Scholar, Division of Medicine, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – drakshatavmc@gmail.com
2M.V.Sc Scholar, Division of Animal Biochemistry, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – kaivalya.deshmukh17@gmail.com
3M.V.Sc Scholar, Division of Animal Biochemistry, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – yashuramya92@gmail.com
Abstract:
Lumpy skin disease (LSD), caused by the lumpy skin disease virus (LSDV), affects cattle and water buffaloes, posing economic risks. Recently reported in India with a 7.1% morbidity rate, LSD exhibits fever, anorexia, and distinctive nodules on mucous membranes, leading to abortion and death. Spreading from the Middle East and Africa, recent cases surfaced in Asian nations like Bangladesh and China. The disease’s current status in India is uncertain. This study highlights recent epidemiological insights, emphasizing transboundary dissemination, disease characteristics, diagnosis, and the potential role of vaccination in controlling LSD.
Keywords: Lumpy skin disease , Transboundary spread , Outbreak , India
Introduction:
Lumpy skin disease (LSD), caused by the non-zoonotic Lumpy Skin Disease Virus (LSDV) of the Capripoxvirus genus, primarily affects cattle and water buffaloes. The disease, also known as Pseudo-urticaria, exhibits vector-borne transmission through biting flies, mosquitoes, and ticks. While natural infection in sheep and goats is rare, experimental infection has induced skin lesions. LSD is characterized by fever, lymph node swelling, skin nodules, leading to emaciation, reduced milk production, and infertility. The economic impact encompasses meat and milk production, hide quality, and reproductive efficiency. LSD is a notifiable disease with global implications for livestock trade. Although endemic in Africa, recent reports extend its reach to various continents, including Asia, with cases documented in China and Bangladesh. The disease’s introduction to India suggests possible livestock or vector movement across borders. This review provides a concise overview of LSD epidemiology, emphasizing the need for effective disease management strategies.
Aetiology:
The Lumpy skin disease virus (LSDV), causing Lumpy skin disease, is a member of the Poxviridae family within the Chordopoxvirinae subfamily. This subfamily, consisting of viruses affecting vertebrates, includes the Capripoxvirus genus, containing sheeppox virus (SPPV), goatpox virus (GTPV), and LSDV, affecting sheep, goats, and cattle, respectively. LSDV is an enveloped virus with a brick-shaped structure, 320 × 260 nm in size, and double-stranded DNA. Its genome, 151 kbp large, includes 156 putative genes with 97% nucleotide identity to SPPV and GTPV. Gene loss, observed in Capripoxviruses, limits host range evolution. The LSDV genome has 146 conserved genes, sharing collinearity with other Chordopoxviruses, while the terminal region exhibits differences in virulence and host range genes. LSDV contains homologous genes found in other poxvirus genera, such as interleukin-10, IL-1 binding proteins, G protein-coupled CC chemokine receptor, and epidermal growth factor-like protein.
Transmission:
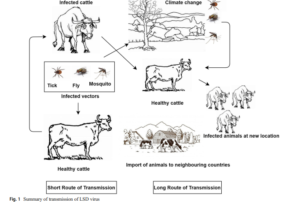
The primary mode of Lumpy skin disease (LSD) transmission is mechanical, facilitated by vectors. In endemic regions like sub-Saharan Africa, Egypt, and Ethiopia, disease incidence rises during seasonal rains and summer, aligning with vector peak activity. Cases decrease in winter and resurge with spring and summer. Despite limited animal movements, transmission occurs up to 200 km away through airborne insect vectors. Insects, particularly ticks (Amblyomma spp., Rhipicephalus decoloratus, Rhipicephalus appendiculatus, Amblyomma hebraeum), flies (Stomoxy calictrans, Biomyia fasciata), and mosquitoes (e.g., Culex mirificens, Aedes natrionus), play a role in mechanical transmission. Direct animal contact has minimal transmission, with the virus secreted in various fluids (milk, nasal secretions, saliva, blood, lachrymal secretions), indirectly infecting animals sharing feeding and watering troughs. Intrauterine transmission and transmission through semen persistence are documented. Iatrogenic transmission through shared needles during mass vaccination is another potential route. Quarantine alone may not suffice, as vector movement can contribute to disease spread.
Host range:
Cattle (Bos indicus and Bos taurus) and buffalo (Bubalus bubalis) are susceptible to Lumpy skin disease (LSD), with Bos taurus being more vulnerable than indigenous breeds. All age groups can be affected, but calves exhibit faster lesion development within 24 to 48 hours. Under natural conditions, wild animals show resistance, although experimental infections have induced clinical lesions in species like Giraffe, impala, Arabian oryx, springbok, oryx, and Thomson’s gazelle. The role of wildlife in LSDV transmission is minimal. Humans are resistant to the virus.
Transboundary spread: Lumpy skin disease (LSD) emerged in Zambia in 1929 and spread across Africa. Increased animal transportation led to infections in the Middle East and Egypt in 1988. The disease re-emerged in 2006 due to unrestricted cattle movement. Transboundary spread continued to Israel in 1989 and later extended to Syria, Jordan, Lebanon, Turkey, Iraq, Iran, Cyprus, Azerbaijan, China, and Bangladesh from 2010 to 2019. Israel experienced a re-emergence in 2019, and India reported its first outbreak in August 2019. Phylogenetic analysis in India revealed genetic closeness to South African strains.
Clinical signs and lesions:
The incubation period for Lumpy Skin Disease (LSD) is 2-5 weeks in natural conditions but 7-14 days experimentally. LSD manifests as acute, subacute, or chronic forms. The disease begins with biphasic fever, and in mild cases, a few painful nodules appear on the skin, muzzle, nares, back, legs, and mucous membranes. Severe cases can have over a hundred nodules, progressing to papules, vesicles, and scabs over 7-12 days. Lesions on mucous membranes and the characteristic “sitfast” holes may lead to secondary infections. Generalized lymph node swelling is common. Histopathology reveals ballooning degeneration and eosinophilic intracytoplasmic inclusions. LSD can result in pneumonia, abortion, infertility, and prolonged recovery due to secondary infections and fly infestation in necrotic lesions.
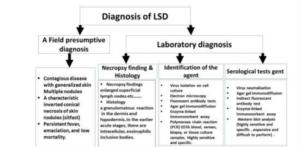
Diagnosis:
Diagnosing exotic diseases like Lumpy Skin Disease (LSD) can be challenging due to unfamiliarity and logistical issues. Clinical signs may resemble other conditions like foot and mouth disease, insect bites, demodicosis, or hypersensitivity. A tentative diagnosis can be based on observed skin nodules, with confirmation through skin biopsy samples transported in a glycerol-phosphate buffer saline medium. Electron microscopy can identify the virus, and histo pathological examination reveals characteristic changes. Agar gel precipitation tests lack specificity. Confirmatory diagnosis involves virus isolation using bovine testes or pre-pubertal lamb cultures. Molecular diagnosis, particularly PCR, is efficient and rapid, with real-time PCR enabling differentiation from other Capripoxviruses.
Prevention and Control:
No effective treatment exists for Lumpy Skin Disease (LSD). Symptomatic treatment involves anti-inflammatory drugs and antibiotics. Control measures include:
- a) Restrict Movement: Strictly prohibit the movement of infected animals to prevent transboundary spread. Quarantine animals with LSD lesions within countries for inspection.
- b) Vector Control: Prevent disease transmission by controlling vector movement through methods like traps and insecticides.
- c) Vaccination: Use live attenuated vaccines based on different LSD virus strains, such as Neethling strain or SIS Neethling type. Vaccines against sheeppox and goatpox, closely related to LSD, can also be effective. Different strains are used as vaccine strains, and long-term vaccination with 100% coverage is crucial for effective control. Calves, pregnant cows, and breeding bulls should be vaccinated regularly, especially before introducing new animals to affected farms.
Conclusion:
Cattle and buffaloes are crucial for the global economy, and Lumpy Skin Disease poses a significant threat to these livestock. While originally confined to African countries, its recent spread to India and other Asian nations raises concerns for agriculture-based economies. The disease’s impact on rural economies and potential reduction in livestock exports make it economically significant. Investigating the entry of LSD into India and implementing effective quarantine, vector control, and vaccination measures are essential for preventing further spread.
References:
1)Brenner J, Bellaiche M, Gross E, Elad D, Oved Z, Haimovitz M, Wasserman A, Friedgut O, Stram Y, Bumbarov V, Yadin H (2009) Appearance of skin lesions in cattle populations vaccinated against lumpy skin disease: statutory challenge. Vaccine 27(10): 1500–1503. https://doi.org/10.1016/j.vaccine.2009.01.020
2)European Food Safety Authority (EFSA), Calistri P, De Clercq K, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Marojevic D, Antoniou SE, Broglia A (2020) Lumpy skin disease epidemiological report IV: data collection and analysis. EFSA J 18(2):e06010
3)Sudhakar SB, Mishra N, Kalaiyarasu S, Jhade SK, Hemadri D, Sood R et al (2020) Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: Epidemiological features and molecular studies. Transbound Emerg Dis. https://doi.org/10.1111/tbed.13579
4) Abutarbush SM, Ababneh MM, Al Zoubil IG, Al Sheyab OM, Al Zoubi MG, Alekish MO, Al Gharbat RJ (2013) Lumpy skin disease in Jordan: Disease emergence, clinical signs, complications and preliminary-associated economic losses. Transbound Emerg Dis 62(5):549–554. https://doi.org/10.1111/tbed.12177
5)Ali AA, Esmat M, Attia H, Selim A, Abdel-Humid YM (1990) Clinical and pathological studies on lumpy skin disease in Egypt. Vet Rec 127:549–550
6)Al-Salihi K (2014) Lumpy skin disease: Review of the literature. Mirror Res Vet Sci Ani 3(3):6–23
7)Biswas S, Noyce RS, Babiuk LA, Lung O, Bulach DM, Bowde TR et al (2019) Extended sequencing of vaccine and wild-type capripoxvirus isolates provides insights into genes modulating virulence and host range. Transbound Emerg Dis 67(1):80–97. https://doi.org/10.1111/ tbed.13322
8)Bowden TR, Babiuk SL, Parkyn GR, Copps JS, Boyle DB (2008) Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology 371(2):380–393. https://doi.org/10.1016/j.virol.2007.10.002
9)Lubinga JC, Tuppurainen ESM, Stoltsz WH, Ebersohn K, Coetzer JAW, Venter EH (2013) Detection of lumpy skin disease virus in saliva of ticks fed on lumpy skin disease virus-infected cattle. Exp Appl Acarol 61:129–138. https://doi.org/10.1007/s10493-013-9679-5.
10)Lubinga J (2014) PhD thesis: The role of Rhipicephalus (Boophilus) decoloratus, Rhipicephalus appendiculatus and Amblyoma hebraeum ticks in the transmission of lumpy skin disease virus (LSDV).
11) Davies FG, Krauss H, Lund LJ, Taylor M (1971) The laboratory diagnosis of lumpy skin disease. Res Vet Sci 12:123–127. https://doi.org/ 10.1016/S0034-5288(18)34204-8
12) King AM, Adams MJ, Carstens EB, Lefkowitz EJ (2012) Virus taxonomy. Classification and nomenclature of viruses. Ninth Report of the International Committee on Taxonomy of Viruses, pp 289–307
13) Gari G, Abie G, Gizaw D, Wubete A, Kidane M, Asgedom H, Bayissa B, Ayelet G, Oura CA, Roger F, Tuppurainen ES (2015) Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine 33:3256– 3261. https://doi.org/10.1016/j.vaccine.2015.01.035
14) Capstick PB, Coackley W (1962) Lumpy skin disease. The determination of the immune status of cattle by an intra-dermal test. Res Vet Sci 3(3):287–291. https://doi.org/10.1016/S0034-5288(18)34901-4
15) Tuppurainen ESM, Venter EH, Coetzer JAW, Bell-Sakyi L (2015) Lumpy skin disease: Attempted propagation in tick cell lines and presence of viral DNA in field ticks collected from naturallyinfected cattle. Ticks Tick Borne Dis 6(2):134–140. https://doi.org/ 10.1016/j.ttbdis.2014.11.002
16) Tuppurainen ES, Lubinga JC, Stoltsz WH, Troskie M, Carpenter ST, Coetzer JA, Venter EH, Oura CA (2013) Mechanical transmission of lumpy skin disease virus by Rhipicephalus appendiculatus male ticks. Epidemiol Infect 141(2):425–430. https://doi.org/10.1017/ S0950268812000805
17) Irons PC, Tuppurainen ESM, Venter EH (2005) Excretion of lumpy skin disease virus in bull semen. Theriogenology 63(5):1290–1297. https://doi.org/10.1016/j.theriogenology.2004.06.013
18) Gumbe AAF (2018) Review on lumpy skin disease and its economic impacts in Ethiopia. J Dairy Vet Ani Res 7(2):39–46. https://doi. org/10.15406/jdvar.2018.07.00187
19) BY-ND CC (2016) EFSA Panel on Animal Health and Welfare (2016). Urgent advice on lumpy skin disease. EFSA J 14(8):e4573. https:// doi.org/10.2903/j.efsa.2016.4573
20) Orlova ES, Shcherbakov AV, Diev VI, Zakharov VM (2006) Differentiation of capripoxvirus species and strains by polymerase chain reaction. Appl Mol Biol 40(1):139–145. https://doi.org/10. 1134/S0026893306010183