Use of Black soldier fly larvae (Hermetia illucens) in Feed for Sustainable Poultry Production in India
Growing demand for food and feed protein
The worldwide demand for protein in food and feed will increase by 1.3-1.5% per year over the next few decades. The total human population will grow to more than 8 billion people by 2030 while meat consumption per capita in developing countries is expected to increase.
Alternatives to soya as feed protein
A major source of feed protein is soya which is mainly produced in North and South America. For several reasons – less genetically modified (GMO) soya, less deforestation, more circular agriculture – EU policymakers and the feed industry are focused on reducing soya imports.
Meat meal:
One alternative could be meat meal. Further to the TSE (transmissible spongiform encephalopathies) issue in the 1980s the EU banned the use of this type of product in animal feeds.
Fish meal:
The use of fish meal as a high-quality protein source has been limited to avoid overfishing of the seas.
Insect meal:
Insects are regarded as an alternative with high potential because the production of insects demands limited amounts of water and land, and they can add value to low-value by-products.
Currently, poultry producers in developing countries are facing problems of high cost and poor quality of poultry feed. Insects are one of the potential protein sources for poultry feed. The use of insects as poultry feed is not in direct competition with human for food consumption. The objective of this paper is to review the current work related to the use of Black Soldier Fly (BSF) larvae meal as an alternative protein source in poultry feeding. Black soldier fly is a harmless insect serving as an alternative protein source in animal feeding and in the disposal of organic wastes, by-products, and side streams.
The single most important fact about Black Soldier Flies (BSF) may be that in the larvae stage, they have the Superman-like ability to transform that waste into high-quality protein. Used as alternative protein additives in animal feed, this translates into an inexpensive, clean and sustainable food source—especially important as farmers, along with global economies, struggle to recover from the financial impact of the Covid-19 pandemic, including food shortages.
Insects have been a part of the human diet for centuries and are currently consumed by humans in many parts of Asia, Latin America and Africa. These are considered to supplement diets of approximately 2 billion people. Due to the current food insecurity situation prevailing in many developing countries and future challenges of feeding over 9 billion people in 2050, lately these have received wide attention as a potential alternate major source of proteins. As a result of increasing incomes, urbanization, environment and nutritional concerns and other anthropogenic pressures, the global food system is undergoing a profound change. There has been a major shift to diets with increased consumption of animal products, and this change is likely to continue in the coming decades. The demand for meat and milk is expected to be 58% and 70% higher in 2050 than their levels in 2010 and a large part of this increase will originate from developing countries (FAO, 2011). The livestock production is resource hungry: for example it occupies 30% of the world’s ice-free surface or 75% of all agricultural land (including crop and pasture land) and consume 8% of global human water use, mainly for the irrigation of feed crops (FAO, 2009; Foley et al., 2011). In addition, the livestock sector contributes approximately 14.5% of all anthropogenic greenhouse gas (GHG) emissions (7.1Gigatonnes of CO2-equivalent per year) (Gerber et al., 2013) and animal products generally have a much higher water footprint than plant-based foods (Mekonnen and Hoekstra, 2012). As a result of huge demand for animal products, enormous need of resources including feeds to produce them will ensue. Fuel-feed-food competition is expected to further exacerbate the situation. A quest for novel feed resources is a must. Insect rearing could be one of the ways to enhance food and feed security (van Huis et al., 2013). They grow and reproduce easily, have high feed conversion efficiency (since they are cold blooded) and can be reared on bio-waste streams. One kg of insect biomass can be produced from on average 2 kg of feed biomass (Collavo et al., 2005). Insects can feed on waste biomass and can transform this into high value food and feed resource. A desk study (Veldkamp et al., 2012) has demonstrated that it is technically feasible to produce insects on a large scale and to use them as alternative sustainable protein rich ingredient in pig and poultry diets, particularly if they are reared on substrates of bio-waste and organic side streams. This paper presents current status on five major groups of insects (black soldier fly, the house fly, mealworm beetles, locusts-grasshoppers-crickets, and silkworm) with regard to their distribution, rearing, environmental impact, nutritional attributes of the insects and insect meal and their use as a component in the diets of ruminants, pigs, poultry (both broiler and laying hen) and fish species, potential constraints, if any in using them as alternative feed resources and future research areas.
Common names
Black soldier fly larvae, black soldier fly larvae meal, black soldier fly prepupae meal, soldier fly prepupae meal, black soldier fly maggot meal
Species
Hermetia illucens Linnaeus 1758 [Stratiomyidae]
Feed categories
Related feed(s)
- Locust meal, locusts, grasshoppers and crickets
- Housefly maggot meal
- Mealworm (Tenebrio molitor)
- Silkworm pupae meal
Description
The black soldier fly (Hermetia illucens Linnaeus 1758) is a fly (Diptera) of the Stratiomyidae family. The adult fly is black, wasp-like and 15-20 mm long (Hardouin et al., 2003). The larvae can reach 27 mm in length, 6 mm in width and weigh up to 220 mg in their last larval stage. They are a dull, whitish color (Diclaro et al., 2009). The larvae can feed quickly, from 25 to 500 mg of fresh matter per larva per day, and with minimal disturbance on a wide range of decaying organic materials, such as rotting fruits and vegetables, coffee bean pulp, distillers’ grains, fish offal, corpses (they are used for forensic purposes), and particularly animal manure and human excreta (van Huis et al., 2013; Diener et al., 2011; Hardouin et al., 2003). In ideal conditions, larvae become mature in 2 months, but the larval stage can last up to 4 months when not enough feed is available. At the end of the larval stage (prepupa), the larva empties its digestive tract and stops feeding and moving (Hardouin et al., 2003). The prepupae then migrate in search of a dry and protected pupation site (Diener et al., 2011). The duration of the pupal stage is about 14 days but can be extremely variable and last up to 5 months (Hardouin et al., 2003). The females mate two days after emerging and oviposit into dry cracks and crevices adjacent to a feed source (Diener et al., 2011). The adults do not feed and rely on the fats stored from the larval stage (Diclaro et al., 2009).
Rearing Hermetia illucens has been proposed since the 1990s as an efficient way to dispose of organic wastes, by converting them into a protein-rich and fat-rich biomass suitable for various purposes, including animal feeding for all livestock species, biodiesel and chitin production (van Huis et al., 2013; Diener et al., 2011). The black soldier fly is an extremely resistant species capable of dealing with demanding environmental conditions, such as drought, feed shortage or oxygen deficiency (Diener et al., 2011). One major advantage of Hermetia illucens over other insect species used for biomass production is that the adult does not feed and, therefore, does not require particular care. It is also not a potential carrier of disease. The larvae are sold for pets and fish bait, and they can be easily dried for longer storage (Leclercq, 1997; Veldkamp et al., 2012). A disadvantage of the black soldier fly for biodegradation is that it requires a warm environment, which may be difficult or energy-consuming to sustain in temperate climates. Also, the duration of the life cycle ranges between several weeks to several months, depending on ambient temperature, and the quality and quantity of the diet (Veldkamp et al., 2012). In aquaculture, using feeds based on black soldier fly larvae open additional marketing opportunities for farmers as some customers are opposed to the use of fishmeal in aquaculture feeds (Tiu, 2012).
Several methods for rearing black soldier flies on substrates such as pig manure (Newton et al., 2005), poultry manure (Sheppard et al., 1994), and food wastes (Barry, 2004) have been designed. Rearing facilities use the migrating behaviour of the prepupae for self-collection: larvae climb up a ramp out of a rimmed container to eventually end in a collecting vessel attached to the end of the ramp (Diener et al., 2011). Optimum conditions include a narrow range of temperature and humidity, as well as a range of suitable levels of texture, viscosity, and moisture content of the diet. Temperature should be maintained between 29 and 31ºC, though wider ranges may be feasible. Relative humidity should fall between 50 and 70%. Higher relative humidity makes the diet too wet, and more generally the diet should have enough structure, otherwise the larvae may have a difficult time crawling on it, consuming it and getting an adequate oxygen supply (Barry, 2004).
It is also necessary to maintain a year-round breeding adult colony in a greenhouse with access to full natural light. The greenhouse must be a minimum of 66 m3 to allow for the aerial mating process (Barry, 2004). Ranges of optimal temperatures, for mating and ovipositing, of 24-40°C or 27.5-37.5°C have been reported (Sheppard et al., 2002). Wide ranges of relative humidity are tolerated: e.g. 30-90% (Sheppard et al., 2002), or 50-90% (Barry, 2004). The greenhouse will need a container with a very attractive, moist medium to attract egg-laying female adults (Barry, 2004).
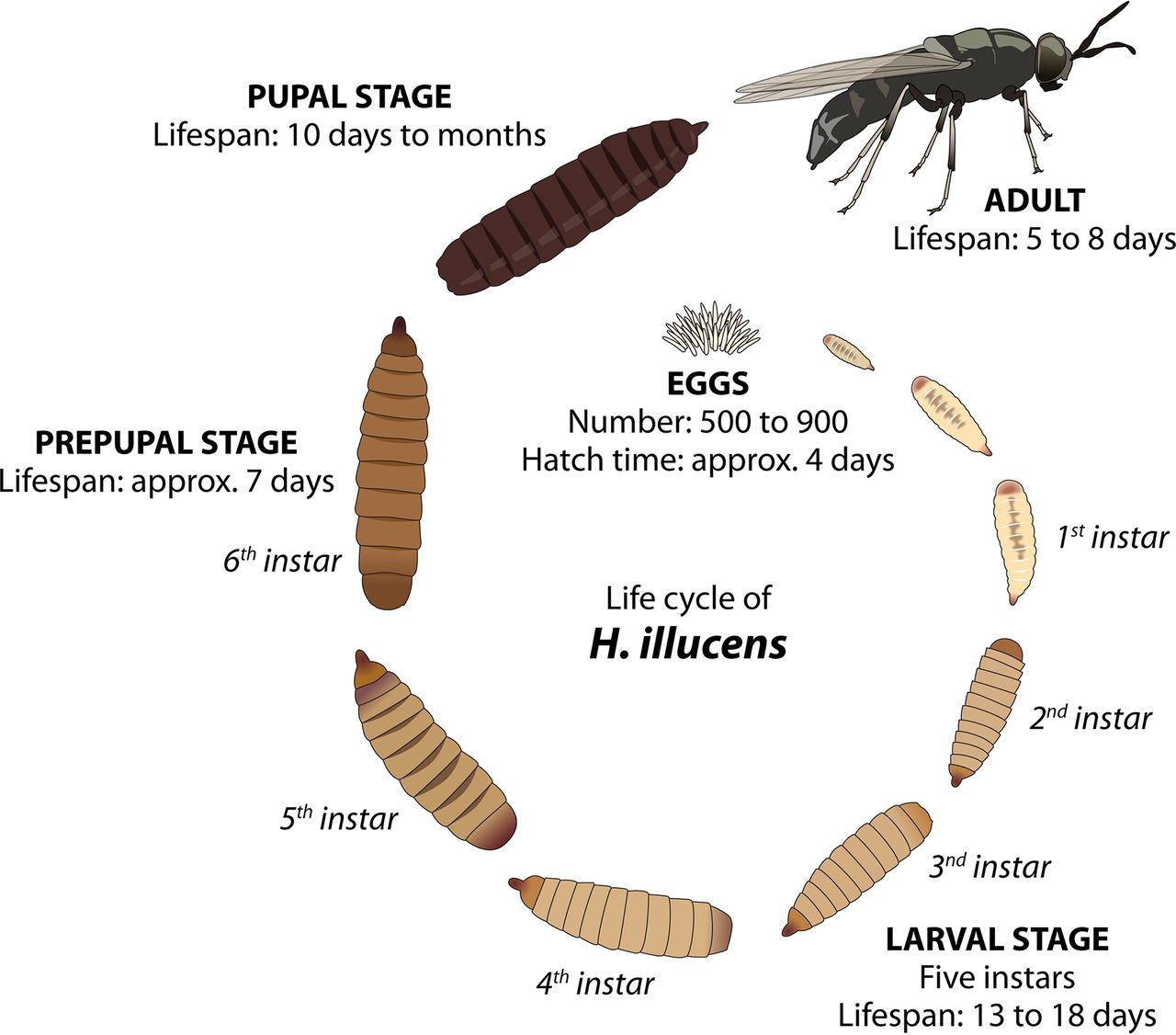
Biology / Lifecycle of Black Soldier Fly
The BSF has five stages in its lifecycle: egg, larvae, prepupae, pupae, and adult. The estimated life cycle of BSF is 40 days but this length differs depending upon the environmental conditions present and the nutrition provided (Alvarez, 2012). Eggs are usually creamy yellow in color and take 4 days to incubate and hatch under optimum conditions of around 20°C to 30°C (Newton 2015). Immediately after hatching, BSF larvae have a dull, whitish color and try to hide away from light due to their photophobic nature (Newton, 2015). Larvae are a very voracious consumer of organic matter and can grow rapidly. The larvae spend most of their life feeding on food and manure wastes and rapidly turn them into fat, protein, and calcium. These nutrients are utilized by larvae to morph into pupae, and further, into adults (Newton et al., 2005). The optimal temperatures of black soldier fly for efficient utilization of feed ranges from 27 to 330C (Alvarez, 2012). Lower temperatures are most likely tolerable because feeding action and metabolism of the larvae will generate some heat which allows their development in colder climates (Newton, 2015). With optimum temperatures, larvae will reach full size (20 to 25mm) in about 4 weeks but can take from 2-4 months if temperature and enough feed are not available ( Newton, 2015). Larvae can tolerate and thrive at densities up to almost 3lb per sq. ft. (14kg/m2 ) (Burtle et al., 2012). Optimal moisture content for the feed ranges from 60% to 90% and is very important for the development of BSF. When the BSF larvae grow into full size (½-inch-long grubs) (Burtle et al. 2012), larvae stop feeding, they become dark and their skin becomes harder. This is the pre-pupa stage, at this point, they start searching for a dry and dark place other than a feed source to pupate and transform into an adult (Newton, 2015). It is supposed that migrating larvae leave chemical trails so that other maggots follow creating a migration path. Along with drier conditions, the pupation site also requires ambient humidity levels of approximately 60% to emerging as adults. Pupation can last 5 to 7 days depend on temperature and ambient humidity. Adults emerge after 10-14 days at 27-36oC (Myers et al., 2008) from their pupae cases. The main purpose of adults in the life cycle is to mate and lay eggs. The adults do not feed but drink water or other liquids if available and rely on the fats stored from the larval stage for their life activity (Newton, 2015). Adults live and mate two days after emerging from the pupal stage (Myers et al., 2008). Female oviposit into dry cracks and crevices near larval habitat (Newton et al. 2005) two days after copulation. A temperature of 25°C-35°C (Newton, 2015)
and ambient light plays a vital role to initiate mating for adult flies, as it is found in the studies that mating levels of adults were highest under natural sunlight. Furman et al. (1959) stated that BSF mating begins in the air with aerial questing after stimulation by light (Alvarez, 2012). The adult flies are photophilic and require strong daylight spectra as well as temperatures between 25°C and 35°C to encourage mating to occur.
Production Systems of Black Soldier
Fly and organic substrates Several methods of rearing BSF have been developed till now. The designs depend on the type of substrate provided for optimal growth and development of BSF. In this type of production system, a conveyor belt made for the collection of waste is designed in such a way that manure solids were collected in the conveyer belt whereas urine plus excess water were drained off the sides of the belt into collection gutters. Then the collected manure solids were delivered to the larval culture basin. The larval culture basin contained 90,000 to 100,000mixed aged larvae/m2 . A 35o ramp along opposing walls of the manure pit is constructed to facilitate the migration of the prepupae to the gutter at the top. This gutter then allows prepupae to pass in collection containers. During this, a portion of the prepupae is saved with the purpose to support the adult soldier fly colony. Eggs from the adult colony are used to maintain larval densities sufficient to digest the manure. The remaining prepupae are frozen until the composite is dried for feed preparation (Sheppard, 2002). Different types of organic waste have been used for farming back soldier fly larvae in confinement. Major BSFL growth parameters such as development time, feed conversion efficiency, mortality, larvae weight, and nutrient composition are strongly affected by the growth substrate (Zheng et al., 2012). Therefore the commercial-scale application of the technology will demand the usage of substrates that can yield quality larvae within a short duration and reduce losses through mortality is necessary. Due to the larvae consuming a wide range of organics, the full range of substrates for rearing BSFL especially for biomass production on a commercial scale are still largely undetermined (Leek, 2017). Organic waste materials are also highly heterogeneous in nature and variable in terms of moisture and nutrient content and therefore generalized applications of findings are almost impossible (Holmes et al., 2012). Nguyen et al. (2015) compared the development rate, size, and mortality of BSFL fed on poultry, feed, pig manure, fish renderings, and kitchen food waste and reported that larvae fed on kitchen food waste had the fastest growth, heaviest biomass, and yields attributed to higher calorie content. Kalova and Borkovcova (2013) fed BSF larvae 14 different waste types over a 14 day period and only four of the waste streams resulted in adult flies during this period among them post- consumer food waste suggesting that these diets were the most suited to larval development.
Distribution
Hermetia illucens is native from the tropical, subtropical and warm temperate zones of America. The development of international transportation since the 1940s resulted in its naturalization in many regions of the world (Leclercq, 1997). It is now widespread in tropical and warmer temperate regions between about 45°N and 40°S (Diener et al., 2011).
Processes
Black soldier fly larvae are used live, chopped or dried and ground. There have been attempts to create a defatted meal by cutting the larvae to enable the leakage of intracellular fat and then transferring the material to a tincture press (Kroeckel et al., 2012).
Environmental impact
The black soldier fly can be used commercially to solve a number of environmental problems associated with manure and other organic wastes. Adult flies are not attracted to human habitats or foods and not considered a nuisance (van Huis et al., 2013).
Biomass conversion
Dense populations of larvae can convert large volumes of organic waste into valuable biomass (van Huis et al., 2013). For instance, larvae can reduce the accumulation of manure from laying hens and pigs by 50% or more without extra facilities or added energy (Sheppard et al., 1994; Newton et al., 2005; Barry, 2004). In Costa Rica reduction values of 65-75% have been observed in field trials with household waste (Diener et al., 2011). In confined bovine facilities, the larvae were found to reduce available phosphorous by 61-70% and nitrogen by 30-50% (Newton et al., 2008).
Odour reduction
Black soldier fly larvae are voracious and process organic waste very quickly, restraining bacterial growth and thereby significantly reducing the production of bad odours. Moreover, the larvae species aerates and dries the manure, reducing odours (van Huis et al., 2013).
Housefly control
Black soldier fly larvae are a competitor to housefly larvae (Musca domestica), as they make manure more liquid and thus less suitable for housefly larvae. Their presence is also believed to inhibit ovipositing by the housefly. For instance, they have been shown to reduce the housefly population of pig or poultry manure by 94-100%. As a result, they can help to control housefly populations in livestock farms and in households with poor sanitation, thereby improving the health status of animals and people since the housefly is a major vector of disease (Sheppard et al., 1994; Newton et al., 2005).
Low pathogenicity
Unlike other fly species, Hermetia illucens is not a disease vector: not only the eggs are never laid on decaying organic material, but, since the adult fly cannot eat due to its lack of functioning mouthparts, it does not come in contact with unsanitary waste materials. Additionally, the larvae modify the microflora of manure, potentially reducing harmful bacteria such as Escherichia coli 0157:H7 and Salmonella enterica (van Huis et al., 2013). It has been suggested that the larvae contain natural antibiotics (Newton et al., 2008).
Effects of BSF larvae on Broilers
The performance of broilers that fed BSF larvae meals was evaluated by different authors. Oluokun (2000) compared BSF larvae with SBM and FM on broiler production. The author suggested that maggot meal could replace FM to upgrade the nutritive value of SBM in the broiler diets without any adverse effect on the body weight (BW) gain, feed intake, and feed conversion ratio (FCR). The feeding of dried BSF larvae as a substitute for SBM resulted in a similar BW gain but a lower feed intake as compared to the control indicating an improved FCR (Makkar et al., 2014). Cousins (1985) reported that broilers fed a BSF-based starter diet showed daily gain and body weight at 10 days old, roughly similar to those fed the fish meal control diet (24.6 vs. 24.5 g/day, 286 vs. 285 g, respectively). These results were consistent with other studies that did not indicate any differences in daily gain or final weight during the grower phase in broiler quails fed either a control diet or BSF larvae meal diet (Cullere et al., 2016). Dabbou et al. (2018) reported that body weight and average daily gain during starter growing periods were increased due to the inclusion of BSF into the broiler chicken diets, while the average daily gain decreased linearly during the finisher stage, which may be attributed to some negative effects of dietary BSF larvae meal on gut morphology when administrated at a high level (10%). In growing broiler quail, Cullere et al. (2016) tested three diets as control, 10% defatted BSF larvae meal (substituted 28.4% soybean oil and 16.1% SBM) and 15% defatted BSF larvae meal (substituted 100% soybean oil and 24.8% SBM).
Effects of BSF larvae on Layers
Maurer et al. (2016)conducted a feeding trial with a partly defatted meal of dried BSF larvae in small groups of laying hens. Experimental diets contained 12 and 24% meals replacing 50 or 100% of soybean cake used in the control diet, respectively. After three weeks of the experiment, there were no significant differences between feeding groups with regard to egg production, feed intake, egg weight, and feed efficiency. There was a tendency (P=0.05) for lower albumen weight in the 24% meal group; yolk and shell weights did not differ. There were also no mortality or sign of health disorders occurred. The DM of feces increased with increasing proportions of meal in the diet, with a significant difference between 24% meal and the control groups (P=0.05). Increases of black fecal pads were observed in the 12% and 24% meal groups. Higher DM of feces and a larger proportion of dark, firm fecal pads with 24% gave reason to assume that in this diet the proportion of meal was too high. The causes of these differences are not fully understood. Hopley (2015) tested the ability of layer hens to be reared on BSF larvae and BSF pre-pupae meal and whether this had any effect on the production parameters and egg quality and concluded that the production parameters were favorable and that the chickens on larvae meal had a lower FCR. Al-Qazzaz et al. (2016) found that the egg quality parameters were either comparable or superior to that of the control treatment and concluded that BSF larvae as a viable protein source for layer hens.In laying hens, the inclusion of 7.5% defatted BSF larvae meal into their diet from weeks 19 to 27 of age showed significantly higher body weight than other groups (Mwaniki et al., 2018). AccordingtoKawasaki et al. (2019) studies on laying hens fed a diet supplemented either with whole (non-defatted) 10% BSF larvae meal or with 10% BSF pre-pupa meal for 5 consecutive weeks did not show significant differences among treated birds and those fed the basal diet. However, Borrelli et al. (2017) reported that the complete replacement of soybean meal by BSF larvae meal in laying hens reduced their body weight (2.09 vs. 1.89 kg, respectively) after a 21-week feeding period. Van Schoor (2017) tested the effect that BSF pre-pupae meal on layer production parameters and egg quality. Results were also positive; egg quality was not affected by the inclusion of the prepupae meal and at the inclusion of 10%, production parameters were also not affected. The rate of degradation (shelf-life) of the eggs was also not affected by the inclusion of the pre-pupae meal. The author concluded that BSF pre-pupae meal may be used as an alternative protein source in layer hen diets with no significant effects on the egg quality, shelf life and production parameters. Mwaniki et al. (2018) also reported that, in laying hens, inclusion of 7.5% defatted BSF larvae meal into their diet from weeks 19 to 27 of age showed significantly higher body weight than other groups.Provision of BSF larvae also had a positive effect on the feather condition of laying hens with intact beaks (Star et al., 2020). Generally, from the above results reported by various authors, it may be concluded that BSF larvae meal could replace FM or SBM in the broiler and laying hen diets without any adverse effect on performance. Up to 25% of housefly larvae can be used in broiler diets without negative effects on performance. The performance is similar to fish meal. Housefly larvae could also replace up to 20% of fish meal in layer feeds without affecting performance and egg quality.
Nutritional Profile of Black Soldier Fly Larvae
The nutritional profiles of BSF larvae as animal feed sources were reported by various authors. The DM content of fresh larvae is quite high (35-45%), which makes them easier and less costly to dehydrate than other fresh by-products (Newton et al., 2008). Maurer et al. (2016) reported that dried full-fat BSF larvae meal contained 41.5% CP, 26.5% EE, 4.3% ash, 0.80% Ca, 0.50% P, 0.08% Na and 0.33% chloride while dried partly-defatted BSF larvae meal consisted 59.0% CP, 11.0% EE, 5.0% ash, 0.98% Ca, 0.63% P, 0.08% Na and 0.28% chloride. The result showed that partly-defatted BSF larvae have better CP, ash, Ca, and P contents than full-fat ones. Newton et al. (2005) found protein levels of 43.2% of BSF pre-pupae reared on pig manure while a value of 42.1% was found when reared on poultry manure (Newton et al., 1977). Relatively, similar protein content (43.6%) was reported by St-Hilaire et al. (2007) when reared on pig manure. Results confirmed by Oonincx et al. (2015) showed CP values ranging between 38% and 46%, and fat values between 21% and 35%. Crude protein and fat values of larvae in a trial conducted by Driemeyer (2016) were 35.9% and 48.1%, respectively. Crude protein content in larvae increased just after hatching, and then it gradually decreased from 4–12 days of larval development, with a minimum concentration of 38% crude protein (CP) at larval phase followed by a further increase of 39.2% in mature larvae on day 14 (Sauvant et al., 2004).The various values of CP contents of BSF reported by authors were due to different types of diet given to the fly and life stage of the fly. According to Newton et al. (1977), the fat content of BSF larvae was 28.0% on pig manure, 35%on cattle manure, and 34.8% on poultry manure. The lipids of larvae fed on cow manure contained21% of lauric acid, 16% of palmitic acid, 32% of oleic acid, and 0.2% of omega-3 fatty acids, while these proportions were, respectively 43%, 11%, 12%, and 3% for larvae fed 50% fish offal and 50% cow manure (Makkar et al., 2014). The BSF larva is a better or comparable amino acid profile to that of soybean meal (SBM) (Tran et al., 2015). The lysine and methionine content of BSF larvae proteins are comparable to that of meat meal (Ravindran et al., 1999). Cullere et al. (2016) reported that the most abundant essential amino acids were valine and leucine, whereas alanine and glutamic acid were rich in defatted BSF larvae meal. The content of amino acids in BSF varies throughout their lifespan and appears to be related to its CP content as the highest level of amino acids contents was mostly expressed in the early stages of larval development then gradually decreased. In dry matter (DM), the adult stage of larvae was characterized by the highest content of amino acids (g/kg) (Liu et al., 2017). Generally, black soldier fly larvae meal CP are comparable to others insect meals and to that of soybean meal but slightly lower than that in fish meal. The BSF larva is high in calcium and phosphorus (Newton et al., 2005). The ash content varied between different samples of BSF pre-pupae depending on their feed substrate. Newton et al. (2005) found an ash content of 16.6% when the BSF pre-pupae were reared on pig manure and 14.6% on poultry manure (Newton et al., 1977). Moreover, an ash content of 15.5% was reported by St-Hilaireet al. (2007) when pre-pupae were fed pig manure. However, a low ash value of 7.8% was recorded by Driemeyer (2016) when BSF pre-pupae were reared on pig manure.
Compiled & Edited by-Dr.Nirbhay Kumar Singh
Assistant Professor
Dept. of Veterinary Anatomy ,Bihar Veterinary College ,Patna
Image courtesy-journals.asm.org
https://www.pashudhanpraharee.com/insects-meal-newer-promising-protein-for-livestock/