Xenotransplantation : An Alternative to Shortage of Donors in Heart & Kidney Transplantation
The first known xenotransplantation was done by the god Shiva. Daksha, the father in law of Shiva, organized a yagna. He insulted Shiva and his daughter. Sati, Shiva’s wife, immolated herself in protest. Daksha’s head was cut off and burnt. Later, when Shiva forgave him, he was brought back to life but with a ram’s head. The more famous decapitation was that of Ganesha. Shiva cut off the head of a baby elephant and transplanted it on to his son’s neck.
For the last 300 years doctors have been trying to replicate this miracle. The process is called xenotransplantation, or the transplanting of non-human organs or cells into a human body.
Xenotransplantation is any procedure that involves the transplantation, implantation or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source, or (b) human body fluids, cells, tissues or organs that have had ex vivo contact with live nonhuman animal cells, tissues or organs. The development of xenotransplantation is, in part, driven by the fact that the demand for human organs for clinical transplantation far exceeds the supply.
Currently ten patients die each day in the United States while on the waiting list to receive lifesaving vital organ transplants. Moreover, recent evidence has suggested that transplantation of cells and tissues may be therapeutic for certain diseases such as neurodegenerative disorders and diabetes, where, again human materials are not usually available.
Although the potential benefits are considerable, the use of xenotransplantation raises concerns regarding the potential infection of recipients with both recognized and unrecognized infectious agents and the possible subsequent transmission to their close contacts and into the general human population. Of public health concern is the potential for cross-species infection by retroviruses, which may be latent and lead to disease years after infection. Moreover, new infectious agents may not be readily identifiable with current techniques.
The Importance of Xenotransplantation
As per the World Health Organization, more than 114,000 organ transplants are performed worldwide yearly, yet they only meet less than 10% of global requirements. If proved compatible in the long term, xenotransplantation might provide an alternate source of organs to patients suffering from life-threatening conditions. This development would assist in easing global organ scarcity.
The abundant supply of organs will aid in the resolution of organ trafficking difficulties as well as ethical concerns about the practice of commercial transactions between a possible receiver and a paid live donor.
HISTORY OF XENOTRANSPLANTATION
Xenotransplantation, a subject of study and experimentation for almost a century, started to receive serious attention from the scientific community in the 1960s as a result of strides made in human-to-human transplantation. Between 1963 and 1993, 31 clinical procedures involving transplantation of solid organs from animal donors were performed in the United States and South Africa. These were extraordinary events. Physicians performed these operations as bridges to maintain life while awaiting a human donor organ.
The first experiments in transplanting chimpanzee kidneys into humans were conducted in 1963 and 1964. One of the patients who received chimpanzee kidneys lived for nine months.
Two of the most publicized xenotransplant operations in the last two decades involved Baby Fae, the infant who received a baboon heart in 1984, and Jeff Getty, an AIDS patient who received a bone marrow transplant from a baboon in 1995. Baby Fae lived with her xenotransplant for 20 days, while Getty rejected the transplanted marrow almost immediately. As of October 1998, Getty remained free of baboon-transmitted viruses and showed no signs of baboon bone marrow in his system.
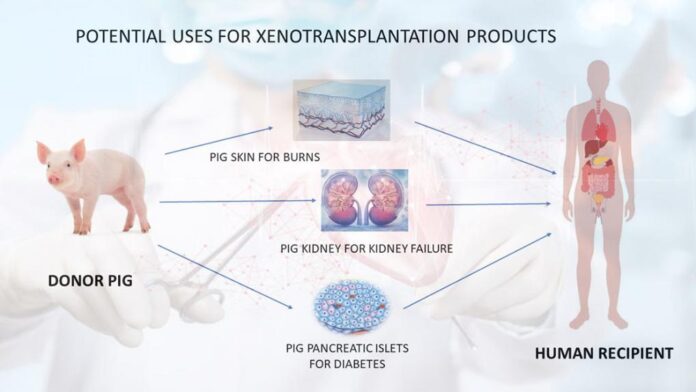
Researchers are currently experimenting with pigs as sources of organs and tissues for xenotransplantation. Studies include the use of pancreatic islet cells and neural cells from pigs for insulin-dependent diabetes and refractory parkinsonism, as well as perfusion of a patient’s fluids through a pig liver situated outside the patient’s body as a temporary strategy to treat liver failure. Patients with Huntington’s disease, which is a neurodegenerative condition characterized by uncontrolled movement and mental deterioration, also are receiving modified tissues from pigs as an experimental treatment. These studies are still very preliminary in testing the safety and effectiveness of this promising treatment.
Between the 17th and 20th centuries, blood was transfused from various animal species into patients with a variety of pathological conditions.
- Skin grafts were carried out in the 19th century on a variety of animals, with frogs being the most popular.
- In 1838 the first corneal xenotransplantation (from a pig) was performed in a patient, whereas the first corneal allograft (human-to-human).
- In 1963–1964, when human organs were not available and chronic dialysis was not yet in use, Reemtsma transplanted chimpanzee kidneys into 13 patients, one of whom returned to work for almost 9 months before suddenly dying from what was believed to be an electrolyte disturbance.
- The first heart transplant in a human ever performed was by Hardy in 1964, using a chimpanzee heart, but the patient died within 2 hours.
- Starzl carried out the first chimpanzee-to-human liver transplantation in 1966; in 1992, he obtained patient survival for 70 days following a baboon liver transplant.
Alexis Carrel working first in France and subsequently in North America, developed surgical techniques for anastomosing blood vessels, which enabled organ transplantation to be carried out successfully for the first time. For this work, he was awarded the Nobel Prize in 1912.
With the advent of genetic engineering and cloning technologies, pigs are currently available with several different manipulations that protect their tissues from the human immune response, resulting in increasing pig graft survival in nonhuman primate models.
Genetically modified pigs offer hope of a limitless supply of organs and cells for those in need of a transplant.
APPROPRIATE ANIMALS FOR USE IN XENOTRANSPLANTATION
Scientists and the U.S. Public Health Service advise that domesticated animals such as pigs and cows be considered as potential tissue and organ sources before nonhuman primates, such as monkeys, for a number of health, safety and logistical reasons. Pigs are preferred because they mature very quickly, produce large litters and have organs of comparable size and function to human organs in both infancy and adulthood. They also can be bred to high health standards in microbiologically controlled environments.
Monkeys, on the other hand, are undomesticated animals that do not fare well in controlled environments and, therefore, it is difficult to raise them to the same high health standards as pigs. Furthermore, their organs are much too small and, like humans, monkeys mature slowly and tend to give birth to one offspring at a time. Although humans might reject nonhuman primate organs less frequently and vigorously than those of other species because of their genetic similarities, these similarities could facilitate disease spread between the donor and recipient. This threat of disease, and ethical issues associated with the use of nonhuman primates as organ sources, have led some government agencies to consider banning the use of nonhuman primates for xenotransplantation. For example, the United Kingdom (UK) has banned the use of great apes and strongly protests the use of other primates for this purpose.
ADDRESSING ORGAN REJECTION
Rejection, in which the recipient’s body attacks the new organ like an infection, is the greatest practical obstacle to xenotransplantation. Traditionally in transplants of organs from one human to another, drug therapies, such as cyclosporine, are used to suppress recipients’ immune systems in order to allow transplanted organs to function without being attacked and rejected as foreign. In xenotransplantation, a more aggressive defense mechanism called “hyperacute rejection” occurs when tissue not recognized as human is introduced to the body. In a matter of minutes, an individual’s immune system sets out to destroy the transplanted organ.
One technology being developed to overcome such organ rejection is the breeding of transgenic pigs. These genetically-altered pigs express specific human proteins that make it more difficult for the human immune system to identify the porcine organ as belonging to a different species. A transgenic pig is bred by injecting a small amount of DNA (or genetic material) mimicking a human gene sequence into a fertilized pig egg and then implanting that egg into a sow leading to the pig’s birth. As demonstrated in recent studies, this technique has addressed hyperacute rejection in nonhuman primates that received organs from transgenic pigs.
New cloning techniques may further enhance the immunocompatibility of pig organs by eliminating the pig gene-products that cause hyperacute rejection. In theory these developments should mean that once transplanted, animal organs could be treated in the same way as human organs, with the use of standard immunosuppressive regimens.
ADDRESSING POTENTIAL RISK OF INFECTION
The transfer of infectious diseases between animals and humans, or cross-species infection, remains an important area of study even though risks have been reduced. In 1997, it was reported that two of four variants of the porcine endogenous retrovirus (PoERV) could infect cultured human cells in test tubes. An endogenous retrovirus is a type of virus that exists as part of the DNA of all mammals and is passed down to offspring over successive generations without causing harm. This earlier report does not indicate if viral transfer would occur as a result of a transplant or whether, if it did happen, it would cause any disease.
Xenotransplantation opponents voice concerns regarding the unpredictable nature of microorganisms. They point to existing human viruses suspected to have originated in animals — human immunodeficiency virus, simian immunodeficiency virus and bovine spongiform encephalopathy (BSE), in which people developed Creutzfeldt-Jakob Disease, the human equivalent to BSE. They express concern that xenotransplantation puts society as well as the individual recipient at risk for disease.
In August 1999, the results of a study were announced which found no evidence of PoERV infection among 160 people who had previously received medical treatment with living pig tissue. A number of patients in the study did show evidence of circulating pig cells, but no evidence of PoERV infection, potentially demonstrating that pig tissue can survive long-term in the human body with no ill effects. Among the patients included in the study were a few individuals who had been pharmacologically immunosuppressed and therefore presumed to be at greater risk of infection. Another study, conducted by the Mayo Clinic, will test pork slaughtering and processing workers to determine whether PoERV is transferred to people who have a history of extensive exposure to swine tissues and fluids. At this time, there is no evidence that PoERV has been transmitted in vivo or that it poses a risk to humans; however, researchers are proceeding with caution to address these outstanding safety concerns. If PoERV’s are found to pose a risk, strategies are being developed that may provide a solution to the problem.
U.S. AND INTERNATIONAL REGULATION OF XENOTRANSPLANTATION
While governments of the United States, the United Kingdom, Spain and elsewhere share similar hopes and concerns regarding xenotransplantation, each is working independently to establish or revise guidelines regarding the regulation of xenotransplantation research. In the U.S., the Food and Drug Administration’s (FDA) Center for Biologics Evaluation and Research published its guidelines in 1999. The guidelines, shaped in part by public dialogue, include long-term monitoring of recipients and the establishment of a registry to archive patient records and donor samples. The various agencies of the U.S. Department of Health and Human Services, including the FDA, the Centers for Disease Control and Prevention and the National Institutes of Health, have encouraged open communication about xenotransplantation at public workshops for several years.
In the United States, several scientists who attended the public conference in January 1998, “Developing U.S. Public Health Policy on Xenotransplantation,” urged the FDA to ban cross-species transplantation research until ethical issues and health risks are resolved. They specifically discussed the potential risk to public health from a viral transfer across species that could result in a new disease epidemic. In April 1999, the FDA released a guidance document stating that any clinical protocols proposing the use of nonhuman primates should include sufficient clinical evidence addressing the risks of such use. At the time of the guidance document, the FDA felt that this evidence did not exist. The FDA decided it was appropriate to allow well-defined and highly controlled clinical trials, which are proceeding at a cautious pace.
The U.S. Department of Health and Human Services is developing several important mechanisms to facilitate participation by the public, scientists and industry in the progress of xenotransplantation. The DHHS is establishing the Secretary’s Advisory Committee on Xenotransplantation (SACX) to review clinical protocols, conduct discussions and make recommendations about the appropriate conditions for use of nonhuman primate organs. In October 1999, the DHHS announced the creation of the SACX and requested nominations for the committee membership. The U.S. Public Health Service is also planning to develop a national patient registry and a biological specimen repository for tissues. The biotechnology industry continues to work closely with the government in the responsible development of regulations and guidelines on the appropriate safeguards for xenotransplantation.
Spain and the UK announced their guidelines in June and July 1998, respectively. Prior to the start of human clinical studies, according to Spain’s regulations, preclinical studies must demonstrate a minimum six-month survival and function period of cells, tissues and organs to be transplanted and the absence of infectious pathogen transmission. People who receive organs, as well as their families and friends, will have to sign informed consent documents indicating that they understand and accept the medical risks and inconveniences involved in these early transplant procedures, such as potential for infections, life-long medical surveillance and potential media attention associated with xenotransplantation. In January 1999, the parliamentary assembly of the Council of Europe called for a worldwide moratorium on xenotransplantation, until technology is evaluated and guidelines are established. A month later, members established a Working Group for the purpose of drafting xenotransplantation guidelines.
The UK’s guidance documents were developed by the UK Xenotransplantation Interim Regulatory Authority (UKXIRA), which is charged to regulate cross-species transplantation on a non-regulatory basis. The guidelines include the following safeguards: the UK Secretaries of State for Health will review each application for a human trial for an organ transplant and make a decision based on advice from UKXIRA; the welfare of animals bred for human transplants will be protected; and the existing Advisory Committee on Dangerous Pathogens will advise on the risks of infection from different types of xenotransplantation therapies proposed.
ETHICAL ISSUES SURROUNDING XENOTRANSPLANTATION
In addition to the critically important potential public health issues in xenotransplantation, there are a number of ethical issues that should be addressed. These include: deciding upon the fairest way to allocate donor animal organs in a society where thousands of people die while waiting for a transplant; deciding whether or not persons who receive xenografts may be compelled to participate in long term follow-up programs because of the theoretical public health risk from endogenous viruses; developing a carefully constructed ethics concerning the creation and care of those animals that will be created to serve as donors; determining when and under what circumstances children and infants may be considered as recipients of xenografts; and studying the potential emotional impact on people of having had their lives prolonged with donor animal organs.
It would be naive to think that all these and other ethical issues will be resolved in advance of the technological readiness to attempt animal to human xenografts. However, it is crucial that those in the biotechnology industry who are working in this area help to initiate and sustain an ongoing public dialogue on these and related issues. BIO is committed to assisting in this process.
How it works
Xenotransplantation is a broad term that includes many different treatment methods. For kidney transplants, the general concept is simple – transfer a working kidney from an animal into a person. Pigs are the most promising source of animal kidneys. This is because pigs:
- are widely available
- have kidneys that are similar in size to humans
- have a low risk of transferring disease
The pigs used for this procedure are raised in a laboratory for this purpose. Their genes are edited to better match human genes. This helps lower the risk of rejection in humans. Rejection is when the body attacks the new organ. The person receiving the xenotransplant will still need to take anti-rejection medications.
Effectiveness
Many studies have been done in animals over the past several decades. Most of these studies transferred pig organs into baboons. Baboons are used because they have a genetic makeup that is very close to humans. These studies showed that transplanting organs across animal species is possible. Clinical trials in humans have not yet started. However, a few operations have been done in rare cases with special permission from the FDA and ethics review boards:
- A person without brain function received two kidneys from a pig. Afterward, he was monitored for 74 hours. The kidneys appeared to continue filtering the blood and making urine. Also, the body did not reject the kidneys.
- An additional two people without brain function also received kidneys from pigs. Afterward, they were monitored for 54 hours. These people had similar results – kidneys continued filtering blood, making urine, and no rejection.
- A severely ill man received a heart from a pig. After the surgery, he lived for two more months. His body never rejected the heart. His exact cause of death is unclear. Doctors found a small amount of a pig-specific virus in the heart after he died. This may have been a factor in his death.
These cases show that it is possible to use some animal organs in humans without causing rejection.
Safety
The most common safety concerns are:
- Infection risk: All transplants come with the risk of infection. Many steps are taken to lower this risk as much as possible. The risk of infection from an animal organ may be higher than the risk from a human organ. This is because the organ may contain animal-specific germs. The risk of these germs spreading among humans is also a serious concern.
- Organ rejection: All transplants also come with the risk of the body rejecting the new organ. Transplants from animals have a higher risk because animals have a different genetic code. To lower this risk, researchers make small changes to the animal’s DNA to better match human genes. Finding the right genetic code to prevent rejection and the long-term impact of these gene edits are important.
- Fulfilling all the kidney’s roles: Early evidence shows that pig kidneys transferred to humans can maintain basic functions. These include filtering waste from the blood and making urine. However, the human kidney has many other important roles. It is not yet clear if transplanted animal kidneys can do all the same things.
Human research is needed to address these concerns. More safety concerns may come up as this research continues, which will also need to be addressed.
Concerns about Xenotransplantation:
Although the potential benefits are considerable, the use of xenotransplantation raises concerns:
- Immunological rejection is a serious concern that patients who undergo animal-to-human transplants face.
- The potential infection of recipients with both recognized and unrecognized infectious agents and the possible subsequent transmission to their close contacts and into the general human population.
- The possibility of retrovirusesinfecting different animals, which could be latent and cause disease years after infection, is a problem for public health.
- Furthermore, novel infectious pathogens can be difficult to spot using present methods (zoonosis).
- Pigs age at a faster rate than humans do because they have shorter lives. As a result, it is uncertain if the organ will continue to function throughout time.
- Animal rights advocates have criticized xenotransplantation for ethical reasons like how they have criticized animal testing.
- Animals’ genetic codes being permanently altered is likewise cause for concern.
Why are pigs the most commonly utilised for xenotransplantation?
- Pigs are becoming more common organ transplant recipients.
- Pigs outperform primates in organ harvesting because they are simpler to grow and reach an adult human size in six months.
- Pigs also have a huge litter. As a result, pigs might produce an endless supply of organs, tissues, and cells.
- Pig organs are anatomically and physiologically comparable to human organs. For example, cardiac output and stroke volume, two important measures of cardiac function, have been observed to be equivalent in pigs and humans.
- Moreover, porcine components are also more suited for genetic engineering. According to scientists, pigs can be genetically modified to lessen the odds of rejection by the human body.
What are the potential advantages of Pigs in Xenotransplantation?
- Pig organs have similaritiesto human organs in respect of anatomy and physiology. For instance, Physiologically, cardiac output and stroke volume, which are major indicators of cardiac function, have been reported to be comparable in pigs and humans, 2. Pigs could provide an unlimited supply of organs, tissues, and cells, e.g., it is easy to raise and achieve adult human organ size in six months from pigs. 3. Pigs are easy to breed and have large litters, 4. From a scientific viewpoint, pigs are genetically modifiable to reduce the chances of rejection by the human body, 5. When bred and housed under ‘clean’ conditions, pigs could provide exogenous infection-free organs, tissues, and cells, For instance, there are now companies breeding genetically modified pigs. One such U.S.-based company, Revivicor supplied the pig heart for the New York transplant, 6. Pigs are produced for food, so using them for organs raises fewer ethical concerns.
Breakthroughs so far
- Pigskin grafts are used on burns.
- Chinese surgeons have used pig corneas to restore sight in 2017.
- Recently, US experts have attached a genetically-altered kidney to a brain-dead person.
Additional Considerations
Xenotransplantation also raises many ethical issues. Below are some of the key issues that have been identified:
- Patient privacy: People volunteering in this research will be monitored for a long time, possibly for life. This can lead the person to feel like their health is on display for others. Family, friends, and other direct contacts may also need to be monitored if there is concern about an animal infection spreading.
- Psychological harm: Some people may not accept a person’s choice to receive a xenotransplant or engage in this research. Sadly, the patient may experience taunting, bullying, or other abuse from others.
- Fair distribution of organs: Using animal organs can increase the number of organs available for transplant. This can shorten the waitlist and increase the number of lives saved. However, how do we ensure organs are offered to patients fairly? Who decides which patient gets a human kidney and who gets one from a pig?
- Animal welfare: These animals are raised in a laboratory. This sterile environment helps decrease the risk of infection for humans. However, this environment may not support the animal’s overall wellbeing. Also, the animal’s genes are edited to lower the risk of organ rejection in humans. Using animals in this way to benefit humans is an ongoing debate.
- Religious and cultural considerations: The most promising source for animal kidneys is the pig. However, some religions and cultures may have strict rules against humans interacting with pigs. Exceptions to save a person’s life usually exist, but still another topic of ongoing debate.
Transplantation in mythology:
Humans have been interested in the possibility of merging physical features from various animal species for hundreds of years.
- The chimera has been used to represent the allotransplantation of organs and cells (transplantation between members of the same species).
- In Hindu mythology, Ganesha is a god with an elephant head and the story also mentions that Ganesha was given a drink “elixir” to Ganesha which could be extrapolated to modern-day induction immunosuppression medicine.
- Sushrutha Samhita provides the first written evidence of forehead flap rhinoplasty, which is practiced today for the reconstruction of the nose.
Future of Xenotransplantation
Xenotransplantation promises that there would no longer be a need for a waiting list, because there would be a ready supply of appropriate organs available at all times, from a genetically modified and carefully overseen population of pigs.
The high cost and complex healthcare infrastructure required for xenotransplantation might limit its use in less affluent countries. This can be resolved in time as the research progresses with substantial success.
Compiled & Shared by- Team, LITD (Livestock Institute of Training & Development)
Image-Courtesy-Google
Reference-On Request.